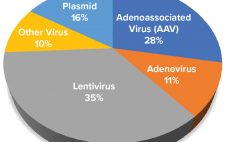
Advanced therapy medicinal products (ATMPs) are engineered to replace defective, disease-causing genes to compensate directly for a genetic defect or to encode a therapeutic protein construct (e.g., chimeric antigen receptor, CAR) for disease treatment. In most instances, a viral vector delivers the engineered genetic payload, targeting cells in situ or ex vivo through cellular modification, expansion, and infusion into a patient. Clinical successes of ATMPs bolstered by regulatory approval of products such as Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), Kymriah (tisagenlecleucel,…
Author Archives: Ashley Fennell
AAV Vector Manufacturing Platform Selection and Product Development
Adenoassociated virus (AAV) vectors have emerged as the prominent delivery mechanisms of corrective gene therapies. Three such products — Glybera (alipogene tiparvovec, uniQure), Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), and Zolgensma (onasemnogene abeparvovec-xioi, AveXis) — have been licensed, and a growing number of candidates are entering late-stage development. In mapping out an AAV gene therapy product development strategy, biomanufacturers should address fundamental considerations for their manufacturing strategies for both phase 1–2 clinical evaluation and translation for commercial market supply. A manufacturing…