From large-scale manufacturing of one-size-fits-all blockbusters to small-scale processing of personalized therapies, the biopharmaceutical industry has undergone a revolution over the past decade. Among the standout milestones is the development of advanced therapy medicinal products (ATMPs). More than 1,000 of these research-intensive therapies are progressing through clinical trials toward potential commercial manufacturing. Cell and gene therapies (autologous and allogeneic) are targeted for many incurable diseases and conditions, including autoimmune disorders and cancers. Despite the excitement about ATMP potential, developers and…
Author Archives: Allan Bream
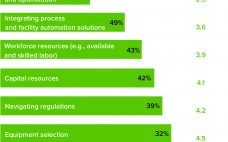
eBook: Trends in Facility Design — In-House Manufacturing Considerations for Cell and Gene Therapy Production
Manufacturing and facility challenges facing cell and gene therapy companies are similar to but more complex than those encountered by companies that produce traditional biopharmaceuticals such as vaccines, monoclonal antibodies, and other therapeutic proteins. A single product can have multiple components, manufacturing of which may or may not be outsourced. Project timelines are short, production technologies are new and evolving, and clinical demands change rapidly. Increasing competition for contract manufacturing services requires reserving capacity far in advance, which in most…