Increasing efficiency and productivity for titer and impurity bioanalytics is even more critical as a result of increasing workloads in supporting multiple programs with limited resources. Current methods such as enzyme-linked immunosorbent assay (ELISA) or high-performance liquid chromatography (HPLC) create bottlenecks in critical workflows because of throughput constraints, repeat testing, or longer assay times. The Gyrolab™ workstation is an automated immunoassay platform that can generate high-quality analytical data at high throughput using nanoliter volumes for more rapid and efficient titer and impurity immunoassays.
Increasing efficiency and productivity for titer and impurity bioanalytics is even more critical as a result of increasing workloads in supporting multiple programs with limited resources. Current methods such as enzyme-linked immunosorbent assay (ELISA) or high-performance liquid chromatography (HPLC) create bottlenecks in critical workflows because of throughput constraints, repeat testing, or longer assay times. The Gyrolab™ workstation is an automated immunoassay platform that can generate high-quality analytical data at high throughput using nanoliter volumes for more rapid and efficient titer and impurity immunoassays.
Gyrolab offers key advantages for applications such as protein expression, cell line development, and the detection of bioprocess impurities. It is a robust, automated platform providing high throughput, enabling data-driven decisions with minimal bench time and rapid time to results.
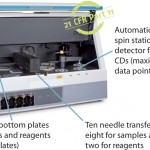
Gyrolab xP Workstation
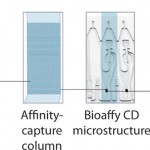
Gyrolab xP workstation is an automated platform that uses proprietary microfluidics technology to perform immunoassays at nanoliter scale. At the core of the Gyrolab system is a compact disc (CD) microlaboratory, with embedded microstructures containing 15 nL streptavidin affinity capture columns. By integrating microfluidics with sensitive fluorescent detection methods and intuitive software, Gyrolab yields quantitative assay data and highly reproducible results for bioanalysis in under an hour.
Gyrolab Bioaffy CD: Miniaturizing Immunoassays
Using an affinity capture flow-through format, Gyrolab allows only brief contact between the immobilized column and the sample, eliminating incubation times. This permits specific affinity capture while minimizing nonspecific binding and matrix effects, which compromise the performance of traditional ELISAs. High-capacity affinity columns with biotinylated capture reagents and fluorescently labeled detection reagents contribute to the consistent outcome of 3–4 logs of dynamic range using Gyrolab for most assays.
| Gyros Platform Benefits Cell Line DevelopmentBroad Dynamic Range: Sensitivity down to 1 ng/mL and broad ranges (3–4 logs) to support posttransfection clone selectionRapid Results: Rapid quantification of murine or human IgG in under an hour to optimize plating density and clone selectionAutomation and Throughput: Reduce manual hands-on time with up to 560 data points generated unattended in under 5 hours
Precision and Robustness: Provides reproducibility for better consistency and easy assay transfer |
Faster Time to Results with Parallel Processing
Miniaturization reduces the cost of critical reagents and results in faster reaction times. This, along with parallel processing of up to 112 data points in 50 minutes under identical conditions, means that Gyrolab improves workflow efficiency, speeds up assay times, and improves precision and reproducibility.
High-Throughput IgG Titer for Clone Screening
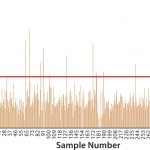
FIGURE 3: High-throughput screening of low titers at early stage cell line development (data courtesy of Merck)
Selection of stable, high-producing clones during cell line development (CLD) is subject to aggressive timelines and produces hundreds of clones requiring rapid assessment for product titer. Figure 3 shows titer distribution of all clones in a screen of low titers at early stage CLD. Posttransfection samples from 96-well plates were measured for IgG titer using a Gyrolab workstation, which processed 332 samples in four hours. Clones with high titers were further expanded.
Minimizing Repeat Analysis of HCP Impurities
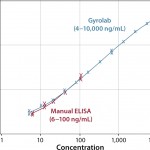
Figure 4 compares the measurement range of Gyrolab with ELISA for an HCP impurity assay and shows 100× broader range. Figure 5 shows correlation with ELISA for samples from various purification steps. Gyrolab system produced broader dynamic ranges, precision, and reproducibility, thereby improving dilutional linearity for minimizing repeat analysis.
| Gyros Platform Benefits Downstream PurificationBroad Dynamic Range: minimizes dilutions and the need for repeat analysis with 3-4 logs of dynamic rangeRapid Results: data-driven decisions with results in under an hourAutomation and Throughput: reduces manual hands-on time with up to 560 data points generated unattended
Precision and Robustness: provides reproducibility for better consistency and easy assay transfer |
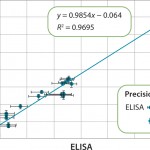
FIGURE 5: Correlation of results for samples throughout the purification of a therapeutic protein are shown. The error bars indicate that the results from Gyrolab xP workstation are less variable. (Data courtesy of MedImmune)
Automating Acid Dissociation for Leached Protein A Analytical Testing
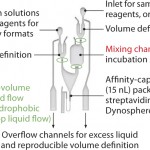
The Gyrolab mixing CD (Figure 6) is a new concept for quantifying residual protein A in the presence of IgG. The CD contains a mixing chamber where acid pretreatment of samples is automated in the Gyrolab workstation before analysis. Figure 7 shows the dose–response relationship for MabSelect™ SuRe spiked into human polyclonal IgG in Rexxip buffer at different pH conditions.
Summary
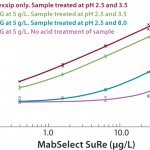
FIGURE 7: Different sample containg MabSelect™ SuRe in IgG at 5 g/L in Rexxip using different combinations of buffers at different pH
Assay miniaturization and automation enable high-throughput screening and analysis of biologic drugs, enhancing productivity across drug and bioprocess development. Demand is growing for rapid, robust, efficient, and reproducible tools and methods for evaluating a product’s purity and titer. Gyrolab’s miniaturized format, fast turn-around time, broad dynamic range, and reproducibility make it an ideal platform to support all phases of cell line development, bioprocess, and production.
References
1 Mou X. Automation Analytical Assays to Support High Throughput in Process Development of Biologics. Bioprocess International Conference, 2013, Boston, MA.
2 Heo J. The Automation of a High-Throughput Residual HCP Assay to Support In-Process Analysis of Monoclonal Antibodies. Gyrolab Seminar, Morristown, NJ, 2012.
3 Roussis et al. High-Throughput Process-Related Impurity Testing Using a Gyrolab Workstation. Well-Characterized Biological Products, Washington DC, 2011.
Joy Concepcion is marketing manager at Gyros, Inc. USA, 1-908-755-0059; joy.concepcion@gyros.com.