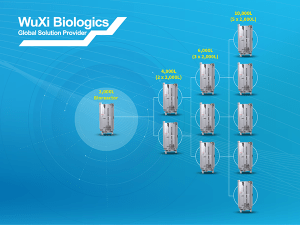
 Changes in production scale required due to increasing product needs throughout clinical trials introduces risk to the product development timeline. Both the process and the product can be dramatically impacted from scaling-up to larger production volumes due to changes in the bioreactor microenvironment. By “scaling-out,” instead of “scaling-up” these risks can be mitigated. The “scale-out” biomanufacturing paradigm, which involves simply adding one or more of the same-sized bioreactor to the manufacturing run to obtain the production volume required, can also efficiently and cost-effectively be applied for commercial scale production.
Changes in production scale required due to increasing product needs throughout clinical trials introduces risk to the product development timeline. Both the process and the product can be dramatically impacted from scaling-up to larger production volumes due to changes in the bioreactor microenvironment. By “scaling-out,” instead of “scaling-up” these risks can be mitigated. The “scale-out” biomanufacturing paradigm, which involves simply adding one or more of the same-sized bioreactor to the manufacturing run to obtain the production volume required, can also efficiently and cost-effectively be applied for commercial scale production.
Here we review, through a Question-and-Answer (Q&A) format, the inherent benefits of this bioproduction strategy as you scale throughout clinical trials and also review the significant cost advantages once in commercial production. Throughout this article we explain why scale-out strategies are ideal for multi-use and multi-product manufacturing facilities, how the strategy utilizes the inherent advantages of single-use bioreactors and we provide strategies for upstream (cell culture) and downstream (purification) scale-out processes during critical late-stage process validation activities.
We then address how a scale-out strategy offers significant advantages during commercial manufacturing, when sudden changes to product demand in either direction occur. The final Q&A addresses that a “scale-out” strategy can apply not only to drug substance manufacturing but also drug product by utilizing advanced modular, fully robotic and programmable fill systems.
