Cygnus Technologies, part of Maravai LifeSciences, is the biopharmaceutical industry’s partner in host cell protein (HCP) and other process-related impurity detection and analytics. In addition, Cygnus now provides innovative viral clearance solutions.
Cygnus helps companies developing therapeutic proteins, vaccines, antibodies, plasma derivatives, and gene therapies ensure the safety of their biotherapeutics before human trials, regulatory approval, and commercial release. Cygnus provides analytical tools and solutions for improving bioprocess development for faster regulatory approval and better clinical outcomes.
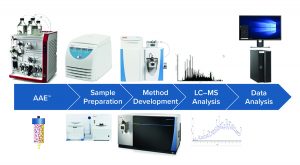
Cygnus is an industry pioneer responsible for developing and commercializing the first generic assay kits for HCP detection. Its reputation for quality is recognized by the industry and global regulatory agencies. Cygnus continues to advance the science of bioprocess impurity detection with new breakthroughs, including its antibody affinity extraction (AAE™) technology and orthogonal methods for HCP analytics that integrate mass spectrometry (MS), AAE™, and ELISA. To reduce the cost and risk associated with viral clearance studies, Cygnus also offers a unique approach that uses noninfectious mock virus particles (MVPs) through its MockV™ virus-clearance kits.
Fill out the form below to read the complete capabilities review and learn more about Cygnus’s offerings for HCP analytics now.