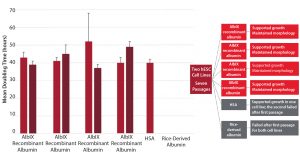
Figure 1: Increasing product shelf life; postthaw stability at 2‚Äď8 ¬įC (study carried out in collaboration with the University of Barcelona and Xcelia of Banc de Sang Teixits SA)
Stem cell therapies are some of the most cutting-edge and sophisticated therapeutic developments. They offer an attractive alternative approach to more widely used treatments for conditions such as multiple sclerosis, metabolic diseases, cardiovascular disease, liver disease, and cancer. But developers still face challenges, some of which can be addressed by the use of recombinant human albumin. As a long-established
ingredient of cell culture media, albumin is well recognized for its ability to facilitate growth of many cell types. The industry is expanding its use of high-quality, fully recombinant, current good manufacturing practice (CGMP) excipients in cryopreservation and formulation of stem cell therapies. Herein we describe the manufacturing, formulating, and handling challenges associated with the development of cell therapies and assess how the use of fully recombinant human albumin in the culture, expansion/differentiation, cryopreservation, and formulation of stem cells compares with alternative approaches.
Cell Therapy and Albumin
Cell heterogeneity is a main concern in¬†cell therapy development and¬†formulation. It is a function of the entire¬†value chain design ‚ÄĒ from harvesting,¬†upstream culture, and cell preservation to¬†therapeutic administration. Consequently,¬†biomanufacturers strive to understand¬†and control all process steps to optimize¬†cell viability and variability.
Serum and human serum albumin (HSA) are useful stabilizers in cell culture and preservation media. In stem cell culture development, albumin contributes different functions. Present at amounts of about 40 g/L, human albumin is the most
ubiquitous protein in blood. It serves as a buffer or reservoir for smaller entities such
as metals, hormones, fatty acids, and toxins, and it shuttles such molecules from areas of high concentration to sites of low concentration. Albumin constitutes about 75% of the colloidal oncotic (colloidal osmotic) pressure of blood, and its single free cysteine makes up most of the reducing equivalents in blood. These properties make albumin a useful component in development of stem cell therapies.
Regulatory concerns over blood-borne contaminants (e.g., mycoplasma, viruses, and prions), potential issues with reliability of supply, and the performance variability of HSA and undefined serum all have led to an increased industry demand for more well-defined, well-characterized, and easily controlled media. Recombinant human albumin is a serum-free, CGMP raw material of high consistency and purity that enables complex culture media to be chemically defined. Regulatory agencies prefer the use of chemically defined media because comprehensive quality information must be available for their constituents.
Recombinant Albumin in Cell Therapy
Cell therapy developers can encounter a number of challenges when optimizing cell performance and controlling variability. From a processing perspective, aggregation, shear, surface interaction growth rate, and reproducibility all can pose serious issues
for manufacturing. Efforts to maintain cell identity and multipotency and to ensure cell survival during transformation also can lead to problems during development. Formulation, quality control of final products, storage and transportation, apoptosis, loss of identity, multipotency, and safety all can be sources of risk to product development.
Recombinant human albumin enhances cell therapy applications, thus making it one of the best ways for cell therapy developers to improve product quality. Albumin readily coats the surface of cells, thus eliminating unwanted interaction between cell and surface. This protein also can prevent aggregation of cells in formulation, protect cells from shear stress during processing, and stabilize cells during freeze and thaw. Most important, albumin does not contain blood-derived impurities that otherwise could activate unwanted cell pathways. And recombinant albumin can help maintain the safety and efficacy of final drug products during storage.
Recombinant Albumin in Stem Cell Cryopreservation
For development of commercial stem cell therapies, cryopreservation is a vital and
often necessary step because it eliminates the requirement for a continuous culture for maintaining viability, which increases flexibility and logistical advantages. Cryopreservation ideally takes place at two stages in a development process leading to a differentiated stem cell therapy dose. The first stage generally is at an upstream point, typically as close to harvest or stem cell generation as possible. The second stage takes place as far downstream in a process as possible, ideally after production of a
differentiated stem cell therapy dose. 
The first step in cryopreservation can contribute economies of scale because multiple batches can be produced either in parallel or in combination, depending on the nature of a therapy. The second step allows for flexibility in distribution and dose administration. Such advantages make cryopreservation highly useful in product development. 
During cryopreservation, stem cells usually are removed from their growth medium (which supports the cells’ biological need for growth), and they are placed in a cryopreserving medium. That environment generally contains only salts and buffers (and no other small-molecule nutrients) to maintain pH and isotonic conditions and different cryopreservation agents such as dimethyl sulfoxide (DMSO) and albumin. After the formulation step, stem cells are quickly frozen. Once thawed, the cells are either
returned to a growth medium supporting further processing or introduced to a final formulation solution, depending on whether they are to be further processed or administered.
Using albumin can provide several unique advantages to a cryopreservation process. Albumin can help stem cells endure media change and other transitions because it covers cell surfaces and enhances the buffering capacity and stability in solution. The purity and source of albumin (plasma-derived or recombinant) play significant roles in
preventing human mesenchymal stem cells (hMSCs) from progressing to late-state apoptosis (Figure 1). That ensures a higher postthaw viability of stem cells
cryopreserved with recombinant human albumin and a longer shelf life than is
achievable with other techniques.
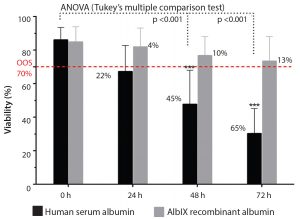
Figure 1 shows the postthaw viability of hMSCs, comparing Albumedix
recombinant albumin (AlbIX) with HSA. With HSA, viability drops significantly below the release criteria of 70%, whereas the AlbIX albumin maintained significantly better cell viability after thawing than HSA. The product met the acceptance criteria after 72 hours,
whereas products conditioned with HSA were out of specification after 24 hours.
Recombinant Albumin in Stem Cell Formulation
The right formulation is critical to reproducibly delivering a functional cell therapy. Typically, formulation takes place immediately after generation or thawing of differentiated cells. Following formulation, several time-consuming release assays must be performed before a product is administered to patients. As a result, the longer that cells can be maintained in a stable state, the greater the applicability and flexibility of a cell therapy.
Preparing stem cell formulations with controlled media generally can make the release of a therapy go smoothly by enabling analysts to focus on the therapy itself rather than the potential effects and impurities from its medium. A controlled formulation should reduce variation in the background of biological assays. However, because of carryover between process steps, it is not enough to use controlled media to generate a final
formulation. If a controlled final formulation is needed, it must be designed into a process sufficiently upstream to ensure that enough dilutions and exchanges have taken place to mitigate risks from uncontrolled substances. So substances such as
recombinant human albumin should be considered and introduced in advance of
final formulation to minimize additional regulatory scrutiny.
Albumedix conducted an in-house study showing that reduced variability of AlbIX recombinant albumin created the same effect across multiple batches (Figure 2). The product also supported growth and outperformed both HSA and another recombinant albumin.
Additional Perspectives
In many cases, cell therapies are modified to alter their function, to activate in the case of immune cells, or to express certain proteins or antigens on their surface. When such modifications are made using viral-vectored gene therapies to deliver recombinant DNA, chimeric antigen receptors, or CRISPR-Cas9 modifications, recombinant albumin may be able to improve the efficiency of such activities. An article by researchers at the University of North Carolina (1) showed that adding human serum albumin before cryopreservation or during formulation improved adenoassociated virus (AAV) vector transduction five- to seven-fold, resulting in a concomitant increase in expressed and active protein.
Those researchers also conducted¬†mechanism studies and suggest that¬†‚Äúhuman albumin increased AAV vector¬†binding to the target cell surface and¬†resulted in faster blood clearance after¬†systemic administration but did not¬†impact AAV infection pathway.‚ÄĚ The¬†implication is that albumin can act as a¬†‚Äúchaperone‚ÄĚ to assist in the interactions
between AAV vectors and cells. It is interesting to note that AAVs enter cells by means of their endosomes, which also is the pathway by which albumin is recycled. Thus, whether such vectors are used to modify cell therapies or to serve as standalone therapeutics, adding albumin can significantly improve the performance of modified cells. It is not clear whether the use of albumin similarly would improve other viral vectors, so further exploration would be warranted. For that effort, fully recombinant albumin would be preferable to HSA in such cutting-edge technologies.
A Safe Alternative
In both upstream and downstream cell suspensions, recombinant human albumin contributes many useful traits in the development of stem cell therapies. It can act as a nutrient carrier to cells to ensure optimal growth conditions, as a stabilizer for cell membranes and coformulated proteins, as a scavenger of free radicals, and as a viscosity moderator. Recombinant human albumin offers a safe solution for optimized cell performance. Its use not only improves the regulatory pathway of stem cell
therapies, but also can considerably improve the cell viability and morphology while controlling batch-to-batch variability through higher tolerance to stress. Such attributes call for cell therapy and stem cell media developers to begin looking at recombinant albumin in a new way and consider its potential for clinical development of cell therapies. It aids in cryopreservation and expansion improvements and has been a critical tool for enhancing the functional performance of cutting-edge stem cell
therapies. Cell therapy manufacturers can benefit from greater intrinsic safety and
regulatory advantages provided by using recombinant albumin.
Acknowledgment
We acknowledge our collaborators Xcelia, a¬†biotechnology company located at Banc de¬†Sang I Teixits, the UAB (University Aut√≥noma¬†Barcelona), and Eva J√łrgensen for her input¬†and expertise in creating this article.
Reference
1 Wang M, et al. Direct Interaction of Human Serum Proteins with AAV Virions to
Enhance AAV Transduction: Immediate Impact¬†on Clinical Applications. Gene Ther. 24(1) 2017:¬†49‚Äď59; doi:10.1038/gt.2016.75.
Phil Morton is science director of bioprocess characterization, and Dr. Daniel Shelly is business development director at Albumedix; albumedix@discoverypr.com; 44-1606-889-194.