Patient preference and a competitive landscape in the parenteral market have fueled the need for convenient delivery systems and a desire for less‑frequent dosing injections. Monoclonal antibodies (MAbs) often have high dose requirements, so they must be formulated at very high concentrations (1). At low concentrations, an antibody solution’s viscosity increases moderately as a function of protein concentration. But at high concentrations (>100 mg/ mL, depending on the molecule), viscosity increases exponentially (2, 3). Thus, a specification for a protein concentration 90–110% of target has little effect on viscosity of a low‑concentration drug product. But the same specification applied to a high‑concentration drug product can lead to significant variability in viscosity within its design space.
Temperature also can influence viscosity. Parenteral drug products are typically stored refrigerated at 2–8 °C. A decrease in temperature will cause viscosity to increase exponentially (4–7). Some studies describe challenges to manufacturability from high viscosity as a function of protein concentration as well as the influence of protein rheology on combination products for parenteral delivery (6, 8–10). However, the effect of temperature on manufacturability of high‑concentration parenteral formulations has not been sufficiently examined.
Materials and Methods
We measured the viscosity of a MAb drug substance at select protein concentrations between 150 mg/mL and 190 mg/mL and at temperatures between 5 °C and 25 °C using a mVROC microviscometer with an attached water bath (Rheosense). By fitting the data in Sigmaplot and plotting them in a three‑dimensional mesh diagram (Systat Software), we generated a concentration– temperature–viscosity profile. We filled four equivalent glass beakers with equal amounts of water, normal saline, formulation buffer, and drug substance (170 mg/mL MAb in formulation buffer), respectively. The beakers were fitted with temperature probes in the center of the liquid and covered with aluminum foil. We stored prepared samples in a cold room overnight to allow thermal equilibration. Subsequently, we placed the samples in a 20 °C incubator and recorded their temperature over the course of 190 minutes.
Results and Discussion
A high‑concentration MAb drug product with a specification for protein concentration of 90–110% around a target concentration of 170 mg/mL can range from 153 mg/mL to 187 mg/mL. Because viscosity is exponentially dependent on protein concentration, it can vary considerably from lot to lot in this concentration specification range. Viscosity increases exponentially with decreasing temperature, so a significant range of viscosity values may be encountered for high‑ concentration MAb drug products when they are stored at 2–8 °C and at ambient temperature of ∼25 °C. Figure 1 shows that range for a formulation of  170 mg/mL ± 10% at temperatures between 5 °C and 25 °C. At the lowest concentration and highest temperature (e.g., 153 mg/mL at 25 °C), viscosity is ∼9 cP. By contrast, viscosity is ∼67 cP at highest concentration and lowest temperature (e.g., 187 mg/mL at 5 °C).
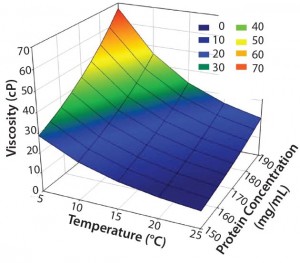
Figure 1: Concentration–temperature– viscosity relationship of a typical high- concentration MAb drug product (5–25 °C and 90–110% of a target concentration of 170 mg/mL)
During drug product manufacturing, a material’s viscosity can directly or indirectly influence certain unit operations. For example, pumping viscous solutions through narrow pathways can generate sufficient shear and cavitation stresses, which could lead to protein unfolding and precipitation (9, 11, 12). That effect can be exacerbated depending on the type of pump used. For example, piston‑driven pumps tend to generate more shear than do peristaltic or diaphragm pumps (10).
Tangential‑flow filtration (TFF) of viscous protein solutions can lead to very high backpressures that may exceed the manufacturing limit of a system or significantly increase processing time. Viscous solutions can be difficult to recover from a TFF system, which can result in substantial product losses (9).
To minimize viscosity at high concentrations, manufacturers can perform sensitive unit operations at or even above ambient temperature. However, if a drug solution is stored under refrigerated conditions between unit operations, then its viscosity can increase significantly. So manufacturers need to map the time it takes for a drug solution at manufacturing scale to return to a temperature that is compatible with an intended unit operation. Mapping the time–temperature profile at scale using actual drug solutions is (in most cases) cost prohibitive. However, aqueous protein solutions generally have a lower heat capacity than water (13), which means that less heat is required to change a protein solution’s temperature than is required for pure water. So it should be possible to map thermal equilibration times using water for injection (WFI) as a worst‑ case scenario rather than using actual drug substance.
To test our hypothesis at laboratory scale, we measured thermal equilibration times side by side for equal amounts of water, normal saline, formulation buffer, and an actual drug substance. Figure 2 shows that the temperatures of drug substance, formulation buffer, and normal saline increased slightly faster than did the temperature of pure water. Those results confirm that WFI can be used as a worst‑case surrogate at manufacturing scale to determine the time required for a drug substance refrigerated between unit operations to return to a temperature that corresponds to a viscosity compatible with an intended unit operation. If feasible, normal saline or formulation buffer can be used for temperature mapping to more accurately reflect the behavior of a drug substance. As another example, we determined that 100 L WFI in a stainless‑steel tank moved from a cold room to ambient temperature required ∼8 h to warm from 5 °C to 15 °C and another 12 h to fully equilibrate to 21–22 °C.
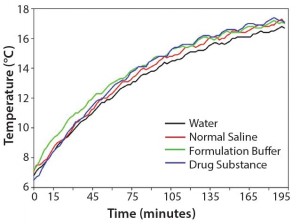
Figure 2: Comparison of the thermal equilibration times of equal amounts (220 g) of water, normal saline, formulation buffer, and drug substance at 20 °C
For a successful manufacturing campaign, a formulation scientist should understand the complete concentration–temperature–viscosity profile of a drug product (and process intermediates, if applicable), viscosity limitations of each unit operation, storage conditions, and equilibration/ hold times between unit operations at manufacturing scale.
Viscosity can influence the administration of a parenteral drug product. With a fixed needle inner diameter and length, the force required to administer a fixed volume in a fixed time interval is directly proportional to the solution’s viscosity. Similarly, if actuation force is fixed (e.g., a spring‑driven autoinjector), then injection time is a direct function of solution viscosity (6).
For a high‑concentration drug product with a concentration specification of 90–110%, even at a fixed temperature, viscosity can vary significantly. The viscosity of parenterals after refrigerated storage is notably higher than at ambient temperature. During the design of a device, those factors must be taken into consideration to ensure that injection forces and times are within acceptable ranges as defined by user requirements. If necessary, equilibration times of a drug product in its final administration device (e.g., prefilled syringe, safety syringe, autoinjector) — from storage temperature to use temperature — should be understood. Where appropriate, design tradeoffs (e.g., needle diameter, actuation means, injection time) must be made to meet usability requirements.
Understanding the Profile
To successfully manufacture high‑ concentration parenterals, manufacturers must understand the concentration–temperature–viscosity profile of their drug products (and process intermediates, if applicable) as well as the limitations of each unit operation. The concentration– temperature–viscosity profile can be determined with little effort and material using a temperature‑controlled microviscometer. However, to determine thermal equilibration and hold times at manufacturing scale, using actual drug substance is not always feasible. Here we have shown that WFI, normal saline, or formulation buffer can be used as cost‑ conscious surrogates for that step.
For successful administration of a high‑concentration parenteral, it is equally important for manufacturers to understand the concentration– temperature–viscosity profiles of their drug product as well as the time requirements to equilibrate those products to temperatures that are compatible with desired administration forces and times.
Acknowledgments
We thank Christina Laskar and Volker Niermann for their valuable input and discussions.
References
1 Warne NW. Development of High‑Concentration Protein Biopharmaceuticals: The Use of Platform Approaches in Formulation Development. Eur. J. Pharm. Biopharm. 78(2) 2011: 208–212.
2 Kanai S, et al. Reversible Self‑ Association of a Concentrated Monoclonal Antibody Solution Mediated By Fab‑Fab Interaction That Impacts Solution Viscosity. J. Pharm. Sci. 97(10) 2008: 4219–4227.
3 Yadav S, et al. Establishing a Link Between Amino Acid Sequences and Self‑ Associating and Viscoelastic Behavior of Two Closely Related Monoclonal Antibodies. Pharm. Res. 28(7) 2011: 1750–1764.
4 Monkos K. Concentration and Temperature Dependence of Viscosity in Lysozyme Aqueous Solutions. Biochim. Biophys. Acta 1339(2) 1997: 304–310.
5 Monkos K. Viscosity Analysis of the Temperature Dependence of the Solution Conformation of Ovalbumin. Biophys. Chem. 85(1) 2000: 7–16.
6 Rathore N, et al. Characterization of Protein Rheology and Delivery Forces for Combination Products. J. Pharm. Sci. 101(12) 2012: 4472–4480.
7 Tang Q, Munro PA, McCarthy OJ. Rheology of Whey Protein Concentrate Solutions as a Function of Concentration, Temperature, pH and Salt Concentration. J. Dairy Res. 60(03) 1993: 349–361.
8 Shire SJ. Formulation and Manufacturability of Biologics. Curr. Opin. Biotechnol. 20(6) 2009: 708–714.
9 Shire SJ, et al. High-Concentration Antibody Formulations. Formulation and Process Development Strategies for Manufacturing Biopharmaceuticals. John Wiley & Sons: New York, NY, 2010; 349–381.
10 Shire SJ, Shahrokh Z, Liu J. Challenges in the Development of High Protein Concentration Formulations. J. Pharm. Sci. 93(6) 2004: 1390–1402.
11 Thomas CR, Nienow AW, Dunnill P. Action of Shear on Enzymes: Studies with Alcohol Dehydrogenase. Biotechnol. Bioeng. 21(12) 1979: 2263–2278.
12 Watterson JG, Schaub MC, Waser PG. Shear‑Induced Protein‑Protein Interaction at the Air–Water Interface. Biochim. Biophys. Acta 356(2) 1974: 133–143.
13 Millero FJ, Ward GK, Chetirkin P. Partial Specific Volume, Expansibility, Compressibility, and Heat Capacity of Aqueous Lysozyme Solutions. J. Biol. Chem. 251(13) 1976: 4001–4004.
Corresponding author Thomas Palm is senior research investigator, Erinc Sahin is senior research investigator, Rajesh Gandhi is group director and Mehrnaz Khossravi is associate director at Bristol- Myers Squibb, 1 Squibb Drive, New Brunswick, NJ 08903; 1-732-227-6129; fax 1-732-227-3764; thomas.palm@bms.com.

