Since the 1985 approval of the first recombinant human growth hormone (hGH, such as Protropin/somatrem human growth hormone from Genentech, now Roche), the number of clinical indications for therapy with hGH has steadily increased (1). That led to a highly successful drug with more than US$3 billion sales in 2011 (2). Even so, hGH shares a common problem with most other first-generation protein therapeutics: a very short plasma half-life of just about two hours in humans. Because such biologics are relatively small molecules, they are rapidly eliminated by renal filtration (3). So they usually have to be injected daily to accomplish the desired therapeutic effect. The same holds true for antibody fragments (4) and for the growing class of alternative protein scaffolds (5). Thus, a technology is needed to prolong their plasma half-lives to meet clinical demands. Indeed, resulting “biobetters” could promise lower dosing with longer time intervals, leading to in improved tolerability and enhanced patient compliance.
PRODUCT FOCUS: BIOPHARMACEUTICALS
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: PRODUCT AND PROCESS DEVELOPMENT, MANUFACTURING, AND FORMULATIONS
KEYWORDS: PROTEIN SECRETION, PLASMA HALF-LIFE, MICROBIAL FERMENTATION, PHARMACOKINETICS, PEGYLATION
LEVEL: INTERMEDIATE
PEGylation technology is an established strategy for extending plasma half-life of small-sized biopharmaceuticals. Through chemical conjugation to polyethylene glycol (PEG), the hydrodynamic radius of a protein becomes larger than the pore size of the glomerular filtration barrier in kidneys, so its circulation time in blood is extended to a therapeutically useful range (6). However, chemically activated GMP-grade PEG can be an expensive raw material, and PEGylated proteins have to be recovered from the reaction mixture by additional purification steps that ultimately lower yields and raise manufacturing costs. Furthermore, the activity of a PEGylated therapeutic protein can be impaired if amino-acid side chains in the vicinity of its active site become modified through PEG attachment (7).
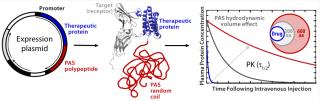
In response to those drawbacks of PEGylation, XL-protein GmbH has developed what we consider to be a competitive technology — called PASylation — that extends the plasma half-life of biopharmaceuticals by applying a natively disordered amino-acid chain as a biological alternative to PEG. A therapeutic protein is genetically fused with a polypeptide sequence comprising several hundred residues of the small amino acids proline, alanine, and/or serine (“PAS”). Like PEG, PAS sequences adopt a random coil structure in aqueous solution, so they generate a large hydrodynamic volume that retards renal filtration of a “PASylated” biologically active protein (8).
Through such means, the typically short plasma half-life of small therapeutic proteins can be prolonged by several orders of magnitude (Figure 1), which allows their dosing frequency to be drastically reduced. PASylation technology offers significant advantages over other methods for half-life extension, especially an overall reduction of manufacturing cost. Because PASylated proteins are directly synthesized as fusion molecules by engineered cells, there is no need to purchase expensive GMP-grade PEG as a raw material. In addition, the fusion protein is expressed as one single product, with no additional coupling and/or separation steps required. Finally, PAS sequences are biodegradable and, unlike PEG, do not accumulate in tissues, which prevents renal vacuolation (7, 9).
PASylated proteins can be conveniently produced by genetically modified Escherichia coli cells. Conventional E. coli production of recombinant proteins (such as for that first-generation hGH product made by Genentech) involves recovery of intracellular inclusion bodies that require protein refolding, a process step commonly recognized as inefficient under typical manufacturing conditions. An alternative is periplasmic production in E. coli (10), which was used, e.g., for Genentech’s second-generation Nutropin/somatropin hGH product. Periplasmic production eliminates the need for refolding because proteins can usually form their correct set of disulfide bonds in the oxidizing milieu of the bacterial periplasm.
However, fermentation yields are typically lower than those from inclusion body processes, most likely due to physical restrictions of the periplasmic space.
Using the proprietary E. coli secretion technology (ESETEC) expression system, Wacker Biotech GmbH surmounts the problems often associated with protein production in E. coli. The company genetically engineered an E. coli K12-derived strain to secrete recombinant proteins directly into the culture medium — across the Gram-negative bacteria’s outer membrane. That greatly simplifies bioproduction and processing because biologically active proteins are recovered directly from culture broth without cell disruption. High secretion efficiency and a lack of spatial constraints posed by the bacterial periplasm provides superior yields of high-quality protein products through a cost-efficient manufacturing process. Because cell disruption and protein refolding are no longer required, primary recovery and purification schemes are simplified, which significantly improves overall process efficiency (11).
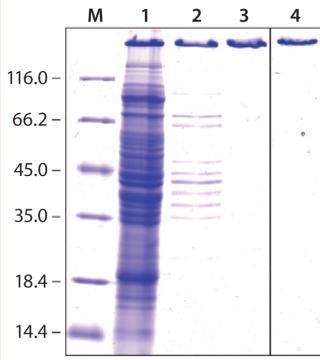
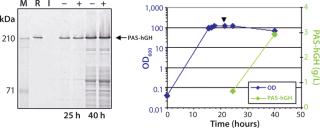
ESETEC Production of PASylated hGHHere, we report results of a feasibility study assessing production of PASylated human growth hormone (PAS-hGH) using the ESETEC technology. We genetically fused a PAS sequence of 600 amino acids to the N-terminus of hGH (normally a single polypeptide chain of 191 amino acids). Furthermore, we equipped the PAS-hGH with an N-terminal His6-tag to facilitate purification using immobilized metal affinity chromatography (IMAC) during the research stage. Recombinant PAS-hGH had a calculated molecular weight (MW) of 73 kDa, but the resulting fusion protein migrated at an apparently much higher MW during sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). That happened because SDS binds poorly to the highly hydrophilic PAS tag (Figure 2). In size-exclusion chromatography (SEC), the fusion protein demonstrated an apparent MW of ∼610 kDa, evidence of the large hydrodynamic volume of the PAS tag (12). PAS-hGH thus can effectively escape renal clearance, and it exhibits a plasma half-life in mice that is roughly 60-fold longer than that of unmodified hGH.
To optimize recombinant protein secretion, we generated several ESETEC strains bearing expression plasmids of different replication origin and encoding PAS-hGH with different signal sequences. Additionally, we tested the coexpression of helper proteins (such as chaperones and disulfide isomerases) from Wacker’s “ESETEC toolbox” to systematically raise the yields of secreted active protein. Multiple cultivation conditions using various temperatures, inducer concentrations (isopropyl-β-D-thiogalactopyranoside), induction time points, and media compositions (including supplementation with L-proline, L-alanine, and L-serine) were systematically scouted as possible methods for improving protein titers in preliminary small-scale cultivation experiments.
We quantified PAS-hGH in cell-free supernatants from centrifuged samples using SDS-PAGE densitometry and a sandwich enzyme-linked immunosorbent assay (ELISA) that detected only correctly folded, biologically active protein. Measured yields of secreted PAS-hGH generally ranged 50–400 mg/L, with the highest levels obtained when expression occurred at 30 °C (data not shown). Some coexpressed helper proteins also had a positive effect on PAS-hGH yields. Notably, adding L-proline, L-alanine, and L-serine to the culture medium had no titer effect. Furthermore, no significant protein degradation was observed; consequently, protease-deficient strains were not included in our study.
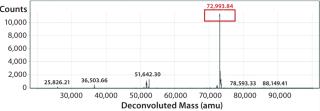
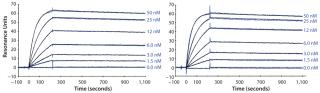
We subsequently purified the secreted PAS-hGH using IMAC and SEC, then analyzed the proteins with electrospray ionization mass spectrometry (ESI-MS). Our protein exhibited an expected MW of 73 kDa and was monodisperse (Figure 3). We also tested the purified protein using real-time surface plasmon resonance spectroscopy (Biacore) for its binding activity to the human growth hormone receptor (hGHR) ectodomain. Results demonstrated that the ESETEC PAS-hGH showed the same binding behavior as a reference material, which was PASylated hGH prepared previously using conventional periplasmic production in E. coli (12).
After confirming that the secreted PAS-hGH was biochemically functional, we cultured the ESETEC producer strain in 3-L bioreactors to further raise product yields. We used the best production parameters (temperature, medium, and induction strength) identified in the small-scale experiments for fed-batch fermentation while testing different induction time points. Remarkably, PAS-hGH titers in cell-free supernatants ranged 2.5–3.0 g/L, verifying that protein yields can significantly increase when recombinant gene expression occurs under the controlled conditions of a bioreactor. We obtained the highest titers by inducing PAS-hGH expression at OD600 = 120, so a late induction point appeared to be crucial.
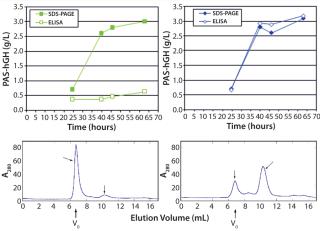
Preventing PAS-hGH AggregationSandwich ELISA tests using PAS-hGH from the small-scale expression experiments revealed that the protein was fully functional. By contrast, although PAS-hGH from bioreactors accumulated in large amounts, it exhibited only a weak ELISA signal, indicating that the protein was mostly present as aggregate and not a correctly folded monomer (Figure 4, TOP LEFT). It is well known that hGH exposes several aromatic side chains in its native state, giving it a pronounced aggregation tendency (13). We confirmed the presence of PAS hGH aggregates in cell-free supernatants from bioreactors using analytical SEC of IMAC-purified material (Figure 4, BOTTOM LEFT).
It has been reported that addition of β-cyclodextrins can effectively prevent aggregation of (conventional) recombinant hGH (13). So we added 5 g/L of a highly water-soluble β-cyclodextrin derivative at the point of induction (OD600 = 120) during fermentation. Unlike the fermentations without it (Figure 4, BOTTOM LEFT), PAS-hGH fermented in the presence of cyclodextrins showed up only in the monomeric SEC fractions (Figure 4, BOTTOM RIGHT).
Otherwise, adding β-cyclodextrin derivative did not interfere with the efficient biosynthesis of PAS-hGH (Figure 5), which was still secreted by the ESETEC producer strain at 3 g/L, as measured by SDS-PAGE densitometry and quantitative ELISA (Figure 4, TOP RIGHT).
It is important that, under those conditions, protein concentration detected by the functional sandwich ELISA perfectly correlated with the SDS-PAGE densitometry data, which indicates that the hormone was biochemically active. We confirmed that using Biacore analysis, revealing a receptor-binding activity of the ESETEC protein that was essentially identical to that of periplasmically produced PASylated hGH (Figure 6). Notably, the ESETEC-secreted PAS-hGH retained a very strong affinity for the hGH receptor (KD = 79.8 ± 3 pM), even higher than what was previously published for recombinant hGH (14). Our results indicate that neither the production system nor PASylation had a detrimental effect on the molecule’s natural binding function.
Furthermore, these results strongly suggest that addition of the β-cyclodextrin derivative prevented aggregation of PAS-hGH without impairing its high yield. Further ESI-MS characterization of the purified protein confirmed the expected MW as well as product monodispersity. We also found that the purified PAS-hGH remained monomeric during downstream processing and storage despite high protein concentration and the absence of cyclodextrin (data not shown).
Discussion and ConclusionsPASylated proteins are generally well suited to production in E. coli because PAS sequences require no glycosylation or other posttranslational modification. Nevertheless, at the onset of our study, we had to consider several obstacles with regard to developing a manufacturing process suitable for industrial-scale production. First, it might have been challenging for E. coli cells to provide sufficient amounts of the amino-acid building blocks (proline, alanine, and serine) required for biosynthesis of PAS polypeptides. Second, we had to demonstrate that the large hydrodynamic volume of the recombinant fusion protein would allow production at high yield. In particular, the protein size could have hampered secretory production in E. coli because the macromolecules do have to cross two cellular membranes to get released into the culture medium. Our results have shown for the first time that PASylated proteins (specifically PAS-hGH), can be efficiently synthesized with a secretory E. coli strain.
PAS-hGH was produced at 3 g/L titers without further fermentation development efforts. Notably, sufficient amounts of the three amino acids required for PAS biosynthesis were endogenously produced by the E. coli cells through their primary metabolism without necessitating medium supplementation. The yield of secreted PAS-hGH we achieved was over 100-fold greater than that from early laboratory experiments using conventional periplasmic production. Consequently, such remarkable titers demonstrate the high potential of the ESETEC approach for large-scale production of PASylated proteins. It is conceivable that such titers could increase further after a fermentation development program.
It is well known that hGH is naturally prone to aggregation (13). We weren’t surprised to observe during our study that the large amounts of PAS-hGH accumulating in the ESETEC culture broth of a bioreactor adversely affected protein solubility. Based on previous information concerning prevention of hGH aggregation with cyclodextrins (13, 15), our addition of a hydrophilic β-cyclodextrin derivative completely suppressed PAS-hGH aggregation in the culture medium. That allowed for recovery of fully soluble and functional PAS-hGH using standard purification techniques.
β-cyclodextrin is acyclic oligosaccharide composed of seven D-glucosyl residues linked by β-(1, 4)-glycosidic bonds. It is torus shaped with an interior that is extremely hydrophobic. Sugar hydroxyl groups oriented to the outside form a hydrophilic surface exposed to solvent. The molecular mechanisms underlying the inhibitory effect of β-cyclodextrins on (PAS)-hGH aggregation are still not fully understood. But compelling evidence suggests that the hydrophobic cavity of cyclodextrins interacts with aromatic side chains on the hGH surface and “shields” part of the molecule’s exposed hydrophobic patches (13, 15), thus effectively preventing aggregate formation.
The ability of cyclodextrins to encapsulate small molecules previously inspired their successful use in pharmaceutical formulations of chemical drugs for parenteral administration (e.g., Pfizer’s Geodon ziprasidone hydrochloride antipsychotic). In fact, cyclodextrins have long been regarded as promising formulation excipients for preventing aggregation of therapeutic proteins (16). It is likely that, in addition to playing a beneficial role during PAS-hGH manufacturing, cyclodextrins could also be used as excipients for pharmaceutical hormone formulations. Our finding that highly concentrated, purified PASylated hGH remained fully monomeric and active after cyclodextrin depletion suggests a stabilizing effect that deserves further investigation. In fact, it may be attributable to the hydrophilic PAS tag.
At a cost of <€1/g, cyclodextrins require minimal investment as raw materials for biopharmaceutical production, so their impact on manufacturing costs is negligible. Their use during fermentation requires no additional process steps for removal because they are efficiently depleted by the chromatography sequence used for purifying the active pharmaceutical ingredient. Altogether, the benefits of using cyclodextrins to stabilize biopharmaceuticals during manufacturing clearly justify the small additional investment in raw materials.
Data provided by this study underpin the high product quality of secreted PAS-hGH. The protein was monodisperse in SEC and MS, an attribute that constitutes a clear advantage over the well-known polydispersity of PEGylated biopharmaceutical products. In addition, N-hydroxysuccinimide–activated PEG is poorly specific in its reaction with lysine side chains, so unwanted by-products are usually formed during the coupling reaction (17). By contrast, PASylated fusion proteins are genetically encoded, so the probability of product heterogeneity (in terms of the PAS modification) is virtually nonexistent. That is especially due to the chemically inert nature of the proline, alanine, and serine residues involved. Even more important, the secreted PAS-hGH retained high receptor affinity, so low doses should be sufficient to elicit a therapeutic effect. Proteins can significantly lose their biological activity after PEGylation, requiring companies to test several PEG variants and coupling conditions during process development to ensure protein functionality (18). PASylation technology can improve product quality while extending the plasma half-life of therapeutically relevant proteins based on a PEG-like biophysical mechanism.
This study provides further evidence that E. coli remains at the forefront in producing novel therapeutic proteins. The ability of ESETEC E. coli cells to secrete a large PASylated protein directly into their culture medium demonstrates the versatility of this workhorse microbe for biopharmaceutical production — even in an era of increasing expectations for biomanufacturing. Moreover, our results reveal a synergy between ESETEC and PASylation technologies. Together, they could ultimately be used for cost-effective production of “biobetters” (e.g., a prolonged-action hGH) both for the benefit of patients and to cut healthcare spending overall. In addition, combining both technologies could boost development of other novel therapeutic protein classes such as antibody fragments or alternative binding proteins.
Author Details
Corresponding author Silvana Di Cesare is business development manager, and Thomas Maier is managing director at Wacker Biotech GmbH, Hans-Knöll-Straße 3, 07745 Jena, Germany. Uli Binder is chief technology officer, and Arne Skerra is chief scientific officer and managing director at XL-protein GmbH, Lise-Meitner-Straße 30, 85354 Freising, Germany. ESETEC is a registered trademark of Wacker Biotech GmbH. PASylation is a registered trademark of XL-protein GmbH. Protropin a
nd Neutropin are registered trademarks of Genentech/Roche.