Increased adoption of disposable storage vessels and mixing systems for biopharmaceutical manufacturing operations has provided economic and efficiency benefits to a number of life-science companies. Single-use technologies have reduced validation requirements, shortened turnaround times, eliminated cleaning regimes, increased the speed of set-up procedures, and facilitated the development of flexible manufacturing platforms. Many biomanufacturers have sought to extend those benefits into the field of cell culture by using disposable bioreactors. Here we describe work undertaken to develop and demonstrate the advantages of the scalable Nucleo bioreactor. Its design is significantly different from that of stainless steel and other single-use bioreactors.
Porous or nonporous microcarriers suspended in a stirred tank or airlift bioreactor are desirable for increasing the available growth area for anchorage-dependent cell lines. Suspending these microcarriers with low sheer stress is an important aspect of the process whether stainless steel or disposable bioreactors are used. The process is controlled by mixing, which can be a defining characteristic of a bioreactor (e.g., the PadMixer mixing technology in a Nucleo system).
PRODUCT FOCUS: ALL BIOLOGICALS, ESPECIALLY VACCINES
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: PROCESS AND CELL CULTURE ENGINEERS, MANUFACTURING, QA/QC
KEYWORDS: BIOREACTORS, DISPOSABLES, VIRUS PRODUCTION, ATTACHMENT-DEPENDENT CELLS
LEVEL: INTERMEDIATE
Most traditional bioreactors are made of stainless steel, which requires extensive cleaning and sterilization after each batch run as well as entailing expensive control validation procedures. To provide a disposable solution for microcarrier cultures, the Nucleo bioreactor was collaboratively developed by ATMI LifeSciences, Pierre Guerin-Biolafitte, and Artelis. We evaluated it for cultivation and infection of MDBK cells (as detailed in the “Materials and Methods” box) immobilized on Cytodex-1 carriers to produce a viral-based veterinary vaccine.
MATERIALS AND METHODS
ATMI Nucleo 25-L single-use bioreactor filled with 15 L of culture medium (MEM +1% v/v nonessential amino acids +5% v/v fetal bovine serum)
Cytodex-1 cell carriers (6 g/L) from GE Healthcare Bio-Sciences (www.gelifesciences.com)
Madin Darby bovine kidney (MDBK) cells
Bovine herpes virus (BHV) for animal vaccine production
Operating conditions: 37 °C, 30 rpm, DO controlled at 50% of air saturation, pH 7.2
Immobilized cell density was estimated after cell lysis with citric acid (0.1 M, 5 min) by counting nuclei with a Guava PCA analyzer (www.millipore.com).
A Disposable Bioreactor System
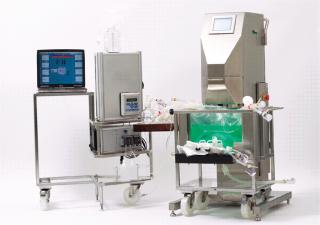
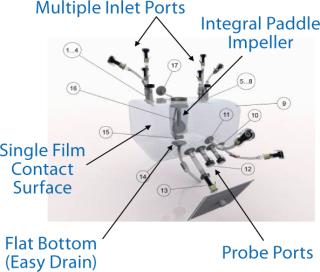
Our experiments involved a 25-L Nucleo bioreactor (also available in working volumes of 50 L, 200 L, 500 L and 1,000 L). This turn-key bioreactor system is based on ATMI’s PadMixer disposable mixing platform with a cube-shaped mixing vessel and paddle-shaped mixing element (Photo 1) that provides efficient mixing and microcarrier suspension at low-shearing rpm speeds. Advanced connectivity enables a full range of processing, monitoring, and control activities without compromising sterility of the culture chamber. The unit is engineered to allow addition of media, cells, and nutrients as well as sampling, monitoring respiration variables, and regulating gas input and exhaust. The system features an integrated Pierre Guerin process software platform for its temperature-controlled tank jacket (Figure 1), mixer, and its paddle-integrated gas sparger (Figure 2). And the reactor vessel features a sealable bottom drain valve, foam trap/vent bottle, connections for bottleor bag-based sampling systems, and a single contact surface to facilitate validation and qualification efforts.
Photo 1:
Protocol and Results
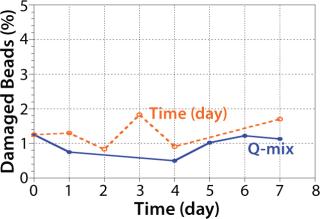
Preliminary Nucleo experiments in culture media without cells confirmed that 30 rpm was sufficient to keep microcarriers in suspension without damaging beads over a period of seven days (Figure 3). The design of the Pad-Drive mixing element enables efficient mixing at low rpm speeds.
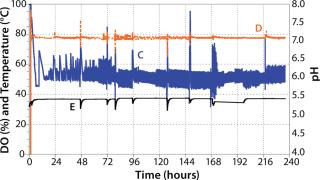
Subsequently, we inoculated 10 MDBK cells per bead in the bioreactor vessel containing 6 g/L of Cytodex-1. During culture, we regulated dissolved oxygen (DO) using pulse oxygen through a 20-m microsparger fixed on the mixing paddle (Photo 2). Using CO2 and NaOH, we maintained the pH between 7.0 and 7.2.
Photo 2:
 gin-top:5px;cursor:pointer;” >
gin-top:5px;cursor:pointer;” >
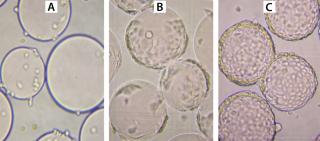
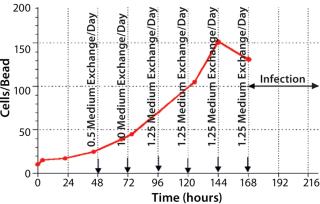
During growth phase, we exchanged the culture medium as necessitated by normal MDBK requirements. After six days of cultivation, immobilized MDBK cells attained confluence on the carriers (Figure 4) at 5.87 × 106 cells/mL — ~150 cells per bead (Figures 5 and 6). After a total change of medium, cells were infected at 168 hours (MOI = 0.15). Resultant virus production was 8.8 × 105, 7.9 × 107, and 1.25 × 108 PFU/mL at 24 hours, 48 hours, and 72 hours, respectively, post infection.
The Nucleo disposable bioreactor provided homogenous and low-shear agitation of MDBK cells while they grew on microcarriers. The sparging system integrated on the mixing paddle provided efficient distribution of gas bubbles and oxygen transfer for the cells. Our experiments demonstrate that the Nucleo single-use bioreactor is suited for cultivation and viral infection of MDBK cells on Cytodex beads, with subsequent harvest of virus at commercially suitable levels. This suggests that the unit presents a viable alternative to conventional stainless steel bioreactors for cultivating adherent cells at high densities MDBK, Madin-Darby canine kidney (MDCK), Vero, and other cell lines have similar growth and cultivation requirements.
Author Details
Corresponding author Roman Rodriguez is global product manager at ATMI LifeSciences, Reuglelstraat 2, B-3320, Hoegaarden, Belgium; 32-1676-8070; roman_rodriguez@atmi.com; www.atmi-lifesciences.com. Samuel Giraud is Nucleo product manager of Global Process Concept SARL, Pierre Guerin-Biolafitte 79 Grand Rue – BP 12, 79210 Mauze, France. And Jose Costillo is president of Artelis SA, Rue de Ransbeek, 310 1120 Brussels, Belgium.