Increased productivity and reduced costs continue to be the driving forces in process development. Recent advances in upstream processes have dramatically improved the productivity of cell culture fermentation. However, prolonged fermentation and high concentration of monoclonal antibodies (MAbs) at harvest may also lead to product degradation and/or aggregation. Clearing these unwanted by-products remains one of the main challenges in downstream processing of therapeutic MAbs.
Media with high binding capacity for target molecules and significant resolution power for impurity clearance are in great demand. Nuvia S — a high-capacity, strong cation exchanger (CEX) built on the industry-proven UNOsphere™ technology — is designed to meet these needs of modern downstream processing.
Flow Properties and Dynamic Characteristics of Nuvia S Media
All UNOsphere-based chromatography media are characterized by their excellent flow properties and fast mass transfer. Nuvia S continues this tradition. The surface extenders grafted into the pore space matrix make the sulfonic acid ligands more available to electrostatic interaction with opposite charges on the incoming antibody molecules.
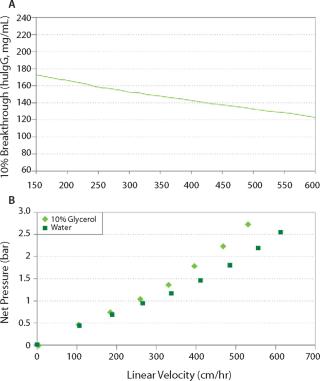
With optimized bead size distribution and mechanical properties, Nuvia S consistently delivers very high binding capacity across a wide range of linear velocities, while maintaining low column pressure during operation (Figure 1). Such performance allows Nuvia S to offer the speed and throughput needed in process manufacturing of MAbs. For initial capture, operation at a high flow rate provides an additional advantage of minimizing the target antibody’s exposure to proteases present in the cell culture feed stream.
Effect of Load pH and Conductivity on Target Molecule Binding By Nuvia S
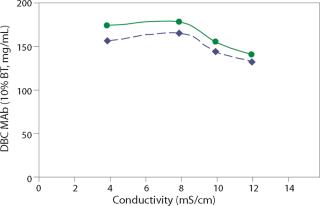
A typical MAb harvest from mammalian expression culture contains a substantial amount of sodium chloride, requiring preconditioning or in-line dilution to reduce harvest conductivity before loading onto a cation exchange column. At pH 5.0, the dynamic binding of MAb G by Nuvia S was tested in the range of 4–12 mS/cm, which corresponds to 15–105 mM of sodium chloride (NaCl) in solution. This cation exchanger’s tolerance to variations in load composition was evident by the fact that the 10% breakthrough binding capacity of MAb G varied only slightly — from 140 to 180 mg/mL — when loaded in the tested conductivity range at 300 cm/hr (Figure 2).
The pI value of an antibody as well as the pH and conductivity of the product pool determine the charge state of both antibody molecules and ligands on CEX media, and hence the strength of electrostatic interaction between them. In light of the interdependence of load pH and conductivity, dynamic binding capacity of a MAb should be determined with multiple pH-conductivity combinations during design of experiments (DoE) process development and optimization.
Removal of Degradation Fragment of MAb R during Capture By Nuvia S
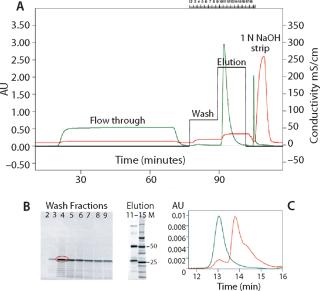
Chromatographic capturing of MAb R from Chinese hamster ovary (CHO) cell culture with Nuvia S is shown in Figure 3A. The predominant protein impurity (circled in red, Figure 3B) was identified by matrix-assisted laser-desorption ionization time-of-flight (MALDI-TOF) mass spectrometry as an L chain fragment of this MAb. This degradation fragment could be selectively desorbed from the column by washing with a low-salt buffer. The intact MAb R was recovered by a salt-step elution. It was found to be free of aggregates or debris by SEC-HPLC analysis (Figure 3C).
Removal of MAb G Aggregates During Intermediate Polishing By Nuvia S
MAb G is a monoclonal antibody that tends to aggregate during the acidic elution step of Protein a affinity chromatography. A salt-step elution method has been developed to recover monomeric MAb G while retaining aggregates on the Nuvia S column until stripping with 1 N NaOH (Figure 4). The overall yield of monomeric MAb G in such a chromatographic preparation was 93%, and the aggregate contamination level was effectively reduced from 13.8% in the load to <1% in the eluate (Figure 4, INSET).
Conclusion
Binding capacity and purification performance are the most important considerations in selecting chromatographic media. The high-capacity, strong cation exchanger Nuvia S is suitable for both capturing and polishing steps in the manufacturing of monoclonal antibodies over a wide range of operating conditions. It can significantly improve productivity by offering the robustness, capacity, and speed for processing large load volumes with smaller columns and less buffer consumption.
Additionally, Nuvia S chromatography is an exceptional tool for removing common product-related contaminants such as MAb fragments and aggregates.
Author Details
Xuemei He, Cheryl Aberin, and Kim Brisack are staff scientists Jiali Liao is a senior staff scientist, Chris Foster is a research associate III, and Mark Snyder is R&D applications manager, all in the process chromatography division of Bio-Rad Laboratories, Inc., 6000 James Watson Drive, Hercules, CA 94547; contact Randy Jacinto, randy_jacinto@bio-rad.com, www.bio-rad.com/process.