Dead-end filters using microporous membranes of synthetic polymers such as PES, polyamide, cellulose acetate, and PVDF are extensively used for microbial control in a wide range of biopharmaceutical liquid filtration applications. Typical applications include sterile media addition into bioreactors, cell harvest clarification, chromatography column protection, and final sterile filtration of purified bulk drug substances. Downstream processing in the biopharmaceutical industry requires multiple bioburden reduction steps that commonly use 0.2-µm sterilizing grade filters.
There are innovative application-specific approaches in sterilizing-grade membrane filtration such as Sartopore® 2 XLG 0.8/0.2 and Sartopore® 2 XLI 0.35/0.2 PES filters from Sartorius Stedim Biotech. Both filters have different prefilter membrane retention ratings designed for optimum performance with fluids having specific particle size distribution characteristics. These filters combine specialized prefilters with optimized pleat pack construction, resulting in 30% higher EFA per 10” element. The 0.2-µm final filter layer is the same as the current Sartopore® 2 0.45/0.2 combination, providing reliable bacterial retention. These filters have reduced total surface area by >50% in some large-scale applications while eliminating the need for prefilters.
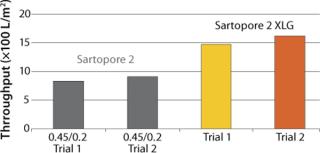
Figure 1 shows V∞ results of testing conducted on small pleated devices for chromatography column protection in a first purification stage, comparing performance of the Sartopore® 2 0.45/0.2 filter with the XLG. The average throughput of the Sartopore® 2 XLG was higher by about 75% in L/m2 and was higher by about 125% when reported in L/10” element.
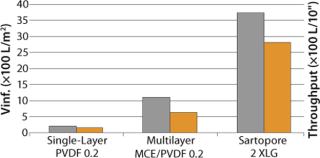
At another customer site, XLG was tested after the first UF step against current single-layer PVDF and triple-layer MCE/PVDF filters. As Figure 2 shows, the V∞ values (gray bars) for the XLG were about 3.4× that of triple-layer mixed cellulose ester/PVDF combination and about 17.5× that of a single-layer 0.22-µm PVDF membrane. The associated throughput values in L/10” (yellow bars) elements would be even higher because of a higher EFA for XLG/10” cartridge.
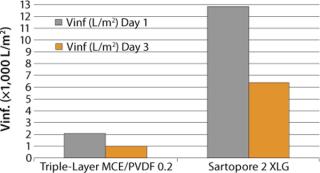
Moving further downstream, Sartopore® 2 XLG and the current triple-layer MCE/PVDF filter performance were compared on an intermediate product pool after viral inactivation (Figure 3). XLG showed V∞ values 6.25–6.5× the triple-layer mixed cellulose ester/PVDF combination. Aging of the pool has a significant effect on the filterability of the solution: V∞ values drop by half at day three compared with day one for both membrane combinations.
In conclusion, Sartopore® 2 XLG shows outstanding performance in many sterile filtration applications in downstream processing. It exhibits as much as 2–6× higher throughput per 10” element, as evidenced here. The combination of the 0.8-µm prefilter membrane and the 0.2-µm final filter membrane turns out to be a very good choice across the breadth of upstream and downstream processes in MAb and therapeutic protein manufacturing.