Profiling of impurities in biopharmaceuticals and their associated intermediates and excipients is a regulatory expectation. Since residuals are typically present at low levels in difficult sample matrices, development and validation of assays can be quite challenging (Figure 1). Matrix types can vary greatly due to the fact that sampling at a variety of process steps is required to accurately monitor throughout the production process.
Some residual impurities are introduced in the upstream steps as required components of the fermentation or cell-culture media. Various impurities result from the culture growth and harvest.
- Nucleic Acids such as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are some of the unwanted cell components that come along with the protein of interest after cell lysis.
- Host Cell Proteins (HCP), like nucleic acids, are also unwanted cell components that come along with the protein of interest after cell lysis.
- Antibiotics are added upstream to the cell-culture media to control bacterial contamination. Common antibiotics used include kanamycin, ampicillin, penicillin, amphotericin B, tetracyline, gentamicin sulfate, hygromycin B, and plasmocin to control mycoplasma.
Some residual impurities are introduced throughout the process
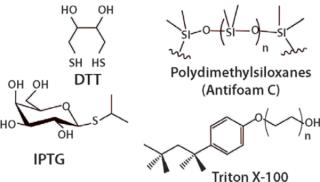
- Process Enhancing Agents/Catalysts are added to make some of the steps more efficient and increase yield of the product. Guanidine and urea are added for solubilization of the fermentation output. Glutathione and dithiothreitol (DTT) are used during reduction and refolding of proteins. Isopropyl -D-1-thiogalactopyranoside (IPTG) is used to induce gene expression and to aid in the refold process.
Other residual impurities are introduced to aid in the purification of the product downstream.
- Chromatographic purification of target proteins may require the use of alcohols and glycols, which must be cleared from the process.
- Surfactants are added to aid in separating the protein, peptide, nucleic acids from the process stream. Examples include Triton-X, Pluronic, Antifoam- A, B, C, Tween or Polysorbate

Analytical Techniques
Mass spectrometry (MS) yields both qualitative and quantitative information and is one of the primary tools for monitoring and identifying residual impurities. The technique consists of ionizing chemical compounds to generate charged molecules or molecule fragments and measurement of their mass-to-charge ratios. In a typical experiment, the sample is introduced to the MS instrument after chromatographic separation (GC or LC). Triple quad mass spectrometry is a powerful tool for monitoring known residual impurities. This type of mass spectrometer allows one to separate a compound, and further break it down to yield highly specific daughter ions, which can be used for quantification and yield high sensitivity and selectivity. Residual antibiotics can be measured accurately at part-per-billion (ppb) levels using LC/MS/MS in very complex sample matrices.
High Performance Liquid Chromatography (HPLC) is one of the most common methods of separation. This technique is best suited for nonvolatile organic compounds. HPLC systems are configured with various detectors including ultraviolet (UV), refractive index (RI), fluorescence, electrochemical, evaporative light scattering detector (ELSD); charged aerosol (CAD), and mass spectrometers (MS). Detectors are chosen based on the analyte of interest, the sample matrix, and the sensitivity; and required selectivity.
Gas Chromatography (GC) is another common method of separation and analysis. Detectors include flame-ionization (FID) and mass spec (MS). The technique is best suited for volatile and semivolatile organic compounds and is commonly used for residual solvent analysis.
Ion Chromatography (IC) is a process that allows the separation of ions and polar molecules based on the charge properties of the molecules. It is a powerful technique best suited for determining low concentrations of ions.
Polymerase Chain Reaction (PCR) is a technique used to amplify a single or few copies of a DNA fragment by several orders of magnitude. Therefore, it is a great technique for confirming clearance of residual DNA. Another form of PCR called reverse-transcriptase or (RT-PCR) can be used for residual RNA.
Enzyme-Linked Immunosorbent Assay (ELISA) is used to detect an antibody or antigen in a sample. There are kits available for host cell proteins specific to a given cell line.
Approaches for Developing Residual Impurity Methods
The first step is to determine how to handle the sample. The protein may first be precipitated out and then the supernatant can be obtained by centrifugation or filtration. Care must be taken to ensure that the residual impurity is not co-precipitated and/or removed with the protein through thorough validation.
The next step may involve further sample preparation including extraction, distillation, and/or cleanup. Extraction approaches may include liquid-liquid extraction with appropriate solvent. Some methods may employ derivatization, which is an approach that modifies the impurity of interest to make it more amenable to a specific detector. Again these steps would need to be evaluated through validation.
Next, the determinative approach must be investigated. For example, a volatile organic compound will most likely be best suited for GC; whereas a nonvolatile compound by HPLC. Also, the detector must be chosen based on the analyte of interest, the sample matrix, the sensitivity and the selectivity required. HPLC with Charged Aerosol Detection (CAD) may be a good approach for compounds that do not respond to UV. Also, if the residual of interest can be ionized, MS/MS is usually a good approach due to its selectivity and sensitivity.
Once these method conditions are established, the method is evaluated for potential interferences and limit of detection, limit of quantitation (LOD/LOQ) within its particular matrix. Also, the method will be tested to ensure acceptable levels of precision, accuracy, and linearity for the intended application. The method then can be used as a qualified method or a protocol could be drafted to perform a formal method validation.
Conclusion
Monitoring of residual impurities encountered in bioprocessing can be quite challenging. Due to the range of potential impurities, many different analytical approaches may need to be employed. Once developed these methods need to be qualified/validated for their intended use.
LANCASTER LABORATORIES: COMPANY OVERVIEW
The global leader in Pharmaceutical and Biopharmaceutical laboratory services, Lancaster Laboratories provides innovative and timely scientific solutions that enable customers to better manage the drug development process.
Pharmaceutical Services
Raw Materials Testing
Release Testing
Microbiology
Facility Validation
Stability Testing and Storage
Impurities Testing
Method Development and Validation
Biopharmaceutical Services
Biochemistry
Residual Impurities
Molecular and Cell Biology
Virology
Microbiology
Cell Bank Manufacturing
Method Development and Validation
Professional Scientific Staffing
Client Facility
Our Facility