Long-term hopes for stem cells to cure various diseases are nourished by the latest developments in stem cell research. First clinical studies have begun (Thera Vitae, Israel: congestive heart insufficiency), and patents have been filed (Stem Cell Therapeutics, Canada: combined regulation for the production of neural cells). Clinical use depends on technological requirements for a reproducible cultivation and controlled differentiation of cells in sufficient numbers, so more and more researchers are focusing on the scale-up of stem cell production. For instance, Susanne Terstegge at the University of Bonn in Germany has studied cultivation conditions on cell growth and differentiation in vitro (1). First results indicate the importance of shear forces and oxygen supply, both of which are discussed here.
PRODUCT FOCUS: C
The primary therapeutic aim in this area is to derive cells from stem-cell populations for the replacement of diseased or damaged tissues. For a personalized clinical therapy, cells must be autologous and produced in sufficient numbers and quality during therapy. For example, mononucleated hematopoietic cells must be expanded at least 2 × 109 in adults during high-dose chemotherapy for treatment afterward (2). Resulting cultivation timeframes can be no more than 7–10 days(3).
Focusing on the next necessary step toward stem cell therapies, researchers are faced with the challenges of process development. Basic research has mainly concentrated so far on aspects such as the influences of cytokines, growth factors, and media compositions in directing the expansion and later differentiation into a desired cell type. Most recent knowledge has led to detailed protocols for the generation of cell lines (leading to, e.g., neural, cardiac, bone, and hematopoietic cells) from embryonic, adult stem, and progenitor cells. This has helped us understand the biochemical mechanisms of cell proliferation and later differentiation in vivo. Every step in that transition is regulated and controlled by the microenvironment of living cells. A direct consequence is that later cell cultivation techniques for generating specific subpopulations must meet the cells’ specific environmental requirements (3).
In comparisons of in vivo and in vitro environments, the “state-of-the-art” cultivation techniques in research do mimic the biochemical, but they cannot and do not replicate normal physiological conditions. Gases, pH, temperature, mechanical stress, and media compositions are rarely taken into account in routine stem cell cultivation despite the fact that oxygen is fundamental for life, and its concentration is an important signal in virtually all cellular processes (4).
Static and Dynamic CulturesVery specialized cultivation methods such as hanging drops do not apply to large-scale production, for which two main stem-cell culture techniques have been established: the static and the dynamic.
Static cultivation is pursued in simple culture systems such as well plates, tissue culture flasks, or gas-permeable culture-bags for easy handling. These are mostly used in incubators to maintain constant temperature and CO2 conditions. Those two parameters are the only ones steered in this method, but like environmental variables such as oxygen tension, pH, and media components they are neither measured nor controlled. Desirable high cell densities within bioreactors can lead to limitations in oxygen and nutrient delivery, which causes variations during static cultivation and therefore makes reliable scale-up difficult if not impossible (3). Additionally, the large quantities of cells needed cannot be accommodated by increasing the of number of tissue flasks, for example, which would lead to a very inefficient and costly labor-intensive process (5).
Dynamic Cultivation: To overcome such disadvantages, several changes have been made to static cultures in the move toward dynamic cultures for higher cell masses. These include perfusion in Petri-dishes to prevent media variations during cultivation (4), uncontrolled spinners for analyzing the influence of shear on cell proliferation (6), and gassing of different atmospheric compositions, just to mention a few. All of these have shown significant improvements in expansion and/or differentiation. But none are suitable for evaluating the concrete influence of a single parameter as other variables change during cultivation.
For reliable scale-up, stirred suspension bioreactors particularly offer the lacking options of static cultures, and they have been successfully tested in the cultivation of hematopoietic cells. Within such bioreactors, adapted impellers agitate culture medium and maintain cells in suspension to provide a homogeneous environment with controlled pH, oxygen, temperature, and other parameters. Suspension cultures are thus much more suitable to scale-up because these bioreactors enable increasing not only cell densities, but also culture volumes without a change of cultivation system (3).
Effects of Physiological Cultivation ConditionsShear: Many stem-cell protocols involve the formation of embryonic bodies (EBs) for cardiac lineages or neurospheres for somatic lineages. Formed under defined conditions, these multicellular aggregates are composed of stem cells and progenitor cells (6, 7).
Many obstacles are faced in the development of a reliable and reproducible protocol for successful cultivation. For example, EBs tend to agglomerate even further toward bigger lumps of cells because of signal proteins such as epithelial cadherin (E-cadherin). Neurospheres that are too large can necrotize inside, damaging cells that surround the necrosis. Consequently, the size of these aggregates is an important factor for cell proliferation and differentiation. Different sizes lead to different microenvironmental conditions in each cell agglomerate, which makes outcomes in cell type and number unreliable.
Agitation should keep cell agglomerates or those in suspension from settling on the bottom of a bioreactor, where they can form a single tissue. But shear force needs to be low enough that it does not damage the stem cells. Corresponding correlations have shown that the optimal shear on a cell aggregate’s surface in suspension culture is sufficient to dislodge some cells (that form new cell agglomerates) without destroying them (5).
In studying neurospheres, Kallos et al. showed that an agitation rate of 100 rpm with a pitch-blade impeller reproducibly produces an optimal sphere diameter of 250 µm (7) Others found an efficient cardiac EB formation by simply optimizing hydrodynamic forces, which led to a high cell density of 12.5 × 106 cells/mL after nine days of expansion and differentiation (6).
Oliver Brüstle, head of the Life and Brain Center at the University of Bonn in Germany, uses small pitch-blade impellers in his microcarrier-based technology for growing stem cells in suspension. Because his group uses cyclic perfusion, it is very important that when the microcarriers settle on the bottom of the vessel, the impeller prevents cells from collecting into large agglomerates. These researchers found DASGIP’s Mini Spinner device to be a useful solution combining small working volumes (40–60 mL) with individual control of shear, oxygen tension, and pH in dynamic cultivation.
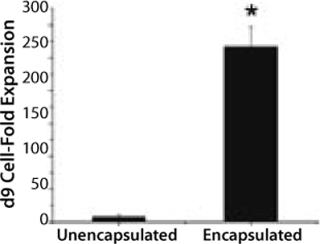
Peter Zandstra and his researchers at the University of Toronto in Canada are following another approach. They seek to prevent agglomeration by inhibiting expression of E-cadherin, which usually triggers the formation of cell aggregates. These researchers examined the addition of a specific antibody to be successful but concluded that scale-up wouldn’t be successful because of the high cost. So they used glass-ball agitators and DASGIP’s control system to develop a new method for more efficient cell expansion. Single human embryonic stem (ES) cells were encapsulated in agarose beads to prevent direct contact among them, and the agitator established a smooth shear regime inside the reactor. Under controlled conditions (constant pH 7.4; constant DO 1%) the beads were expanded to high cell masses before differentiating to a desired cell type. After nine days of proliferation and differentiation, these encapsulated cells showed a 25-fold higher expansion than single cell cultures (Figure 1).
The measurement and control of such cultivation parameters enables fundamental insight into the metabolic changes within ES cells and therefore leads to optimal cultivation conditions, especially in the case of differentiation towards neural progenitor cells. Zandstra has stated, “We use the DASGIP bioreactor system as an effective tool for stem cell bioprocess engineering. It allows us to run several experiments in parallel and gives us good control of microenvironmental parameters that can impact stem cell fate.”
It turns out that the type of cultivation method differs with each cell type. In optimizing results, the need arises for a flexible cultivation system that can be adapted to each stem cell line. The modular DASGIP system provides researchers with a tool (e.g., to test various impeller-reactor combinations) for finding the perfect match for their studies.
Oxygen: Fundamental for life, oxygen (O2) is the most common substrate for cell cultures. But high O2 concentrations are toxic (4). On a tissue level, oxygen tension is considerably lower than the general ambient conditions of 21%. Arterial blood shows an oxygen tension of about 12%, and the mean level of oxygen in tissues is about 3%, with considerable local and regional variations. For example, in bone marrow, oxygen concentrations of 1–6% have been described (3).
Those numbers are valid for adult organs and tissues, and in embryos the concentrations are even lower. Obviously, O2 plays an important role in stem cell metabolism. Such cells normally reside in a particularly low-oxygen niche of the bone marrow, which indicates that O2 may be an important determinant of stem cell mobilization from marrow in disease processes (4).

DASGIP AG (WWW.DASGIP.COM)
Almost all stem cells cultivated at lower, physiologic oxygen tensions proliferate more than in traditional incubators supplied by room air buffered with 5% CO2 (4). O2 levels of 1% support, for example, the proliferation of colony-forming cells (3) and enhance size and number of hematopoietic colonies at 1–10% (5). Even the proliferation of mature granulocytes and hematopoietic cells has been optimized at low oxygen levels, reaching a sevenfold expansion in cultures maintained with 4% O2 (2). Low oxygen concentrations also involve less-reactive oxygen species, lessening apoptosis within stem cell cultures. Nevertheless, it has also been shown that at such low oxygen tensions, sometimes stem cells respond with no expansion at all, whereas at certain higher concentrations their proliferation was accelerated (5). So the mechanisms of oxygen influence on stem cell proliferation and differentiation are still incompletely understood.
When research states that variations in oxygen around cultured cells induces diverse transcriptional and protein-level responses reflective of responses to O2 in vivo, great care must have been taken to control the oxygen tension in vitro. But most research into the effects of oxygen tension on proliferation and differentiation are pursued while maintaining a gaseous overlay of different O2 concentrations inside an incubator, without any actual measurement of the oxygen tension in situ. The above-mentioned desirably low levels of oxygen are a challenge for bioprocessing, in which the oxygen content of media has to be measured precisely and controlled exactly to prevent oxygen limitations.
For cell growth, oxygen transfer is adjusted by increasing the oxygen concentration in the gas phase or by heightening the gas flow rate. This can be enabled by DASGIP’s MX4/4 gas-mixing station, which blends four different gasses (air, O2, CO2, and nitrogen) to provide an individual mixture for each reactor. The actual oxygen tension (e.g., normoxic or hypoxic) is measured in situ and then controlled by the O2 concentration
in the atmosphere above the medium, which allows for reproducibly tight ranges in vitro. Together with the best choice of media compositions, oxygen tension control can be used to direct proliferation and differentiation of stem cell cultures.
pH: The metabolic activity of cells is strongly affected by pH because the activity of their regulating enzymes relies on conformable changes in the reactive centers of such molecules because of alkaline and acidic groups within them. In vivo pH is carefully regulated by ingenious mechanisms, which can be affected by carbon dioxide, lactate production, and many serum substances (especially salts). Growth and differentiation of stem cells is strongly pH-dependant as well. Some differences have been seen in the survival and differentiation of hematopoietic progenitors into granulocyte macrophages (optimal pH 7.2–7.4, the normal pH of blood) and erythtroid lineages (optimal pH ∼7.6) (5). Many studies have shown that medium acidification inhibits growth. Cells of the erythroid lineage are even strongly inhibited when pH dips below 7.1. Below an acidic pH of 6.7, no differentiation or proliferation has been observed at all.
To prevent such inhibitions of proliferation and differentiation during cultivation, the pH of stem cell cultures must be controlled carefully. Most such cultures performed in static or noninstrumented bioreactors are maintained in a constant CO2 and O2 atmosphere and lack any type of pH control(2). That leads to pH shifts during cultivation because of secreted acidic metabolites such as lactate (3). To overcome this disadvantage, it is useful to look at modern cultivation techniques in mammalian cell culture. A closed feedback loop allows tight control of pH within the closest ranges. Comparing in situ measurements with desired set-points leads to changes in the atmosphere of a bioreactor. DASGIP’s gas-mixing station can then adjust the amount of CO2 flushed through a reactor to control pH. The resulting high precision of concentration and mass flow makes this perfectly suitable for stem cell cultivation.
Control Is KeyResearch has shown that culture conditions have an enormous influence on the proliferation and differentiation of hematopoietic (stem and progenitor) cells. Even small changes in oxygen tension, pH, or medium composition can cause significant changes in differentiation patterns and the proliferation potential (3). Attempts to untangle the effects of acid (such as lactate) production, pH, medium use, and differences in oxygen tension on stem cell cultures have proven that each parameter seems to influence cell response (5). In combination with the small starting volumes in early stage cultures, this presents a great challenge for stem cell researchers (3).
DASGIP helps scientists define the right cultivation solutions for their stem cell proliferation and differentiation tasks. Modularity in hard- and software, together with the reactor design itself, should serve as the versatile tool for flexible stem cell research.