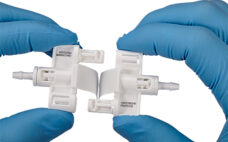
Small-volume biopharmaceutical processes continue to grow as more biopharmaceutical, cell therapy and gene therapy companies develop products. A 2021 Association for Regenerative Medicine (ARM) report states that there are 1,085 cell, gene and tissue-based therapy developers worldwide. These companies are engaged in many small-volume (<10L) processes including early-stage drug development and autologous therapies. The increase in small-volume processes coincides with the ongoing need to create fast, reliable aseptic closed systems. Historically, few convenient options have existed to facilitate sterile processing.…
Thursday, April 14, 2022 Daily Archives
Advanced therapy CDMO roundup: Deals for WuXi, Resilience, Fujifilm
Resilience will make gene therapies for Opus; WuXi Advanced Therapies will make CAR-Ts for Chimeric; and Fujifilm will make RNA oncology candidates for Chimeron. First up in our roundup of advanced therapy contract manufacturing deals this week is Opus Genetics, which has struck a deal with (National) Resilience to support its pipeline of adeno-associated viral (AAV) vector-based gene therapies for inherited retinal diseases. The contract development and manufacturing organization (CDMO) will provide process and analytical development, quality control testing, and…