The US FDA has temporarily paused its inspectional activities to ensure the safety of its staff and the firms it regulates due to the spread of the omicron variant. The Omicron variant, which was first discovered in November 2021, has already caused global disruption, despite reports from the UK suggesting the risk of hospitalization is about one-third of the Delta variant. With case numbers surging, the US Food and Drug Administration (FDA) decided to implement specific temporary changes to its…
Thursday, January 6, 2022 Daily Archives
Univercells adds DNA synthesis through SynHelix buy
Univercells has entered the synthetic biology space through the acquisition of French DNA synthesis firm SynHelix. The deal, financials of which have not been disclosed, sees Belgian bioprocess firm Univercells add technology aimed at simplifying biotherapeutic development through a scalable and automated DNA synthesis platform. SynHelix’s technology is based on the GMP production of long DNA fragments in large quantities and has been touted as an alternative to DNA amplification on bacteria in the synthetic biology, gene therapy, and diagnostics…
Thermo Fisher buys recombinant protein maker PeproTech for $1.85bn
Thermo Fisher has opened its M&A war chest early in 2022, buying biopharma reagent firm PeproTech for $1.85 billion. The deal, announced on December 30, closed yesterday, adding Cranbury, New Jersey-based reagent maker PeproTech to Thermo Fisher Scientific’s cell culture media offering. Founded in 1988, PeproTech offers over 2,000 cytokines, antibodies, ELISA, and cell culture media kits for the development and manufacturing of biologics and cell and gene therapies. According to Thermo Fisher’s CEO Marc Casper, the acquisition “will be…
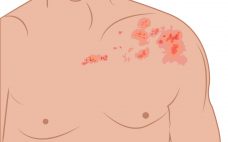
Pfizer and BioNTech to develop first mRNA shingles vax
In Pfizer and BioNTech’s third mRNA collaboration, both firms aim to develop an improved vaccine for shingles using their respective technologies. BioNTech and Pfizer first partnered on infectious diseases in 2018, teaming up to develop a messenger RNA (mRNA) based influenza vaccine. However, it was their second collaboration BNT162b2 (Comirnaty) that saw success, becoming the first mRNA COVID-19 vaccine to receive emergency use approval in December 2020. Now, both firms have teamed up again to produce a vaccine for shingles (herpes…