Providing consistency in cell culture media biomanufacturing is critical to supply continuity. Central to this is the development of redundancy and harmonization across a global manufacturing network. These unprecedented times have also highlighted the importance of strategizing for increased and unexpected demand. Read this Special Report to learn about the importance of equivalency and the strategies used to maintain this critical requirement at Gibco cell culture media manufacturing facilities. Fill out the form below to read the complete report and…
Friday, December 18, 2020 Daily Archives
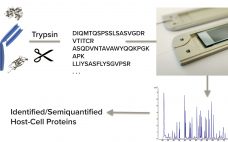
Nontargeted HCP Monitoring in Downstream Process Samples: Combining Micro Pillar Array Columns with Mass Spectrometry
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely. Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high…
eBook: Ion-Exchange Chromatography for Modern Biopharmaceutical Purification
Understanding the functionalities of chromatography resins can improve the product yield and purity in a biotherapeutic purification workflow. Ion-exchange (IEX) chromatography separates biomolecules on the basis of charge. For several reasons, it is the most widely used separation tool for purification of biopharmaceutical products. IEX is a well-characterized purification method with high binding capacity and flexible selectivity. It also works with mild operating conditions that help to preserve the biological activity of a biopharmaceutical drug substance. That versatility enables several…
Fragment-Based Screening in Drug Discovery: How to Improve Hit Rates & Deliver Higher-Value Targets
Learn about the basics of fragment-based drug discovery (FBDD), one of the most valuable approaches to small molecule screening and now even more widely used today by pharma companies than high-throughput screening (HTS) HTS’s main limitations — low success rates for more challenging targets, high level of false positives, and the size of the compound libraries — are propelling the use of FBDD. This change is also driven by the rise in the number of explorative targets, which demands not…
SK Holdings set to jump into cell and gene CDMO space with Yposkesi buy
Korean investment firm SK Holdings is looking to buy Yposkesi to add a cell and gene therapy business to its growing CDMO portfolio. While the deal is not done and financials have not been revealed, SK Holdings is looking to become the majority shareholder in the majority shareholder of Yposkesi, a France-based contract development and manufacturing organization (CDMO) specializing in advanced therapies. If it goes through, Yposkesi will fall under SK’s CDMO holding company, California-headquartered SK Pharmteco, formed last year…