The stakes are high in biologics development, especially in host cell protein (HCP) analysis. Regulatory bodies have rules and suggested strategies for HCP analysis and reporting, and it is likely that removing HCPs is a key concern in your process development. Ultimately, the accuracy of monitoring and success of purifying your biologic of these HCPs can make or break your product. Get it wrong and it could delay your development cycle. In this blog, we outline strategies to optimize your…
Friday, October 16, 2020 Daily Archives
Tech used for new Ebola drug key to COVID-19 fight, Regeneron
Regeneron says rapid response technologies pioneered to combat Ebola are helping accelerate efforts to create COVID-19 countermeasures. Last week Regeneron won US FDA approval for Inmazeb (atoltivimab, maftivimab and odesivimab-), its treatment for Zaire Ebola. The product is made from three monoclonal antibodies that target the Ebola glycoprotein, preventing the virus binding and infecting cells. Regeneron created Inmazeb using the same rapid response manufacturing platform it is currently using for RGEN-COV2, its COVID-19 treatment candidate. Shawn Lawrence, senior director, cell…
Ensuring Everything is A-OK at AKBA
Accurately and reliably producing pharmaceuticals and biotherapeutics is one of the most important – and challenging – tasks in the world of manufacturing. Any misstep in the production process will lead to the creation of a substandard end product that can do substantial harm to the user. That’s why the processes used in these production chains – things like inline buffer formulation (IBF) and virus filtration – must be completed with no deviation from accepted norms. For Asahi Kasei Bioprocess…
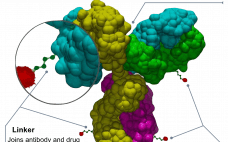
CDMO selection ‘the last barrier’ to ADC advancement
The complex and multiple parts of antibody-drug conjugate (ADC) manufacture means it is integral to choose a CDMO with mAb, small molecule, and bioconjugation expertise, say WuXi Biologics and WuXi STA. The onslaught of ADCs is a hardy perennial in the pages of this publication and others when it comes to predicting the future of medicine. In 2011 when Adcetris (brentuximab vedotin) – then only the second ADC – was approved, it was predicted to be the watershed moment and…