The Novartis gene therapy business will contribute its technology and dedicated space at a facility run by CDMO Catalent to help produce a COVID-19 vaccine being developed by Massachusetts-based researchers. Massachusetts Eye and Ear and Massachusetts General Hospital have teamed to develop a vaccine candidate that uses an adeno-associated virus (AAV) vector to deliver and express the Spike gene of the SARS-CoV-2 virus, which causes COVID-19, to elicit an immune response. The developers have turned to AveXis, the gene therapy division…
Thursday, May 28, 2020 Daily Archives
Optimizing Your Excipient Screening for Vaccine Formulation with an Ultra-Pure Pharmaceutical Gelatin
This webcast features: Jeroen Geeraerts, Business Development Manager, Biomedical, Rousselot The world is working at an unprecedented pace to develop a safe and effective vaccine to combat the COVID-19 pandemic. Currently, five candidate vaccines are in clinical evaluation, and many more are in preclinical testing. Different types of vaccines are being developed using multiple strategies and platforms. Among them are several inactivated-virus and live-attenuated–virus candidate vaccines. As an excipient, gelatin is a key component in many vaccine formulations. Well-known examples…
Challenges and Benefits of Networking Process Control Manufacturing Systems: Integration to Business Systems in Industry 4.0
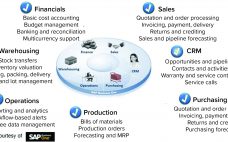
Networking of manufacturing process control systems can lead to benefits of efficiency, increased productivity, and better facility use, leading to lower cost of goods (CoG). Furthermore, integration of manufacturing systems with manufacturing execution systems (MESs) upward to an enterprise resource planning (ERP) business system improves overall organizational efficiency. An ERP system enables optimization of intrafacility manufacturing resources. For multiple manufacturing facilities, it facilitates optimization across a company’s manufacturing network. Enabling Technologies and Systems At the enterprise resource planning level (Figure…
Designing the Right Strategy for Digital Transformation: How a Pragmatic Approach to Digital Transformation Can Help Biomanufacturers Adapt to a Challenging Future
Although the biopharmaceutical industry has enjoyed explosive growth over the past three decades, it still faces an assortment of challenges. Those include growing portfolio complexities, increased demand volatility, stringent regulatory requirements, increased pricing pressures, and growing technological complexities, all leading to severe pressure on profit margins. To overcome such pressures, biopharmaceutical operations need to become more reliable and agile, and they must realize efficiency gains in both manufacturing and supply chains. Digital transformation offers strong value opportunities, including a potential…
Fluorescent Nanosensors: Real-Time Biochemical Measurement for Cell and Gene Therapies
Cell and gene therapies are destined to transform the methods by which global healthcare challenges are approached and overcome (1). The US Food and Drug Administration is reviewing and approving an increasing number of cell and gene therapy products (2), and biopharmaceutical developers are dedicating immense resources to realizing the enormous potential of these therapeutics. Therefore, technologies that facilitate their effective and efficient manufacture will accelerate cell and gene therapies’ transition from medicines of the future to medicines of the…
Soft Sensors for Bioprocess Monitoring
Achieving the high process efficiencies and optimization of Manufacturing 4.0 will require sophisticated software systems, mathematical modeling, and on-line process monitoring. Soft sensors are valuable tools that enable users to measure process parameters in real time. I spoke with Benjamin Bayer, data scientist at Novasign GmbH and doctoral candidate at the University of Natural Resources and Life Sciences in Vienna, Austria, about the potential of soft sensors for bioprocessing and important considerations for their use. Introduction How would you describe…
Merck exercises option to use Vaxxas vaccine delivery patch
Merck & Co. will use Vaxxas’ delivery patch technology in one of its vaccine candidate development programs. Brisbane, Australia based Vaxxas announced the agreement today, explaining the US drug firm exercised an option under a co-development accord signed in 2012. Vaxxas CEO David Hoey told us the technology – known as the High Density Microarray Patch (HD-MAP) platform – “uses an ultra-high density array of very short projections applied briefly to the skin to rapidly deliver vaccine to the abundant…
With $60m in hand, Gamida looks to build out cell therapy infrastructure
Gamida Cell will initiate a BLA submission for lead cell therapy omidubicel later this year and grow inhouse manufacturing capabilities to support production of cancer candidate GDA-201. Israeli cell therapy company Gamida Cell raised $60 million (€55 million) last week through a public share offering. The money will be used to support the approval application and manufacturing capabilities of its cell therapy products, the firm said on an investor call last week. Lead candidate omidubicel – formerly known as NiCord…