Two years after announcing plans to expand into the US, WuXi Biologics has signed a land deal allowing construction of a $60 million plant in Worcester, Massachusetts. For Chinese contract development and manufacturing organization (CDMO) WuXi Biologics, 2018 was a landmark year, signaling major growth through the announcement of several expansions. Along with several additional single-use biomanufacturing plants in China, the firm set its sites on global expansion, first announcing plans for a 54,000 L plant in Ireland, then a…
Monday, May 25, 2020 Daily Archives
AZ aims to ship Oxford’s COVID vaccine from September using BARDA’s $1bn
AstraZeneca says $1 billion from the US Biomedical Advanced Research and Development Authority (BARDA) will fund production of Oxford University’s SARS-CoV-2 vaccine. The Anglo-Swedish drug firm said it would begin production of the vaccine candidate – previously known as ChAdOx1 nCoV-19 and now called AZD1222 – in the autumn. It added that the BARDA funding will also support a support a Phase III trial involving 30,000 participants and a pediatric study. AstraZeneca set the funding as part of an effort to…
Why, Why, Why… ELISA? A Look at the Benchmark HCP Assay
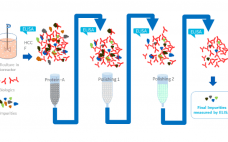
Host cell proteins (HCPs) are a primary source of impurity in biologics manufacturing. When present in drug formulations, HCPs can reduce efficacy, introduce toxicity, and increase risk of long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are critical when developing a new biological drug, both for ensuring patient safety and fulfilling regulatory guidelines. HCP populations can be complex and structurally diverse, and most changes in upstream culture conditions affect HCP concentrations and control strategies. Accurate and reliable HCP…