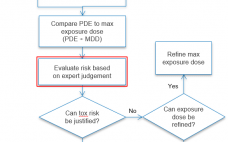
This webcast features: Dr. Sherry Parker, Senior Director, Regulatory Toxicology and Technical Services, WuXi AppTec Extractable and leachable studies of single- and multi-use manufacturing components should be followed up with a chemical safety assessment to understand the chemical risks based on the toxicity of the chemical, the dose and the exposure duration of the drug product, and the patient population for its intended use. This case study will illustrate considerations when conducting a chemical safety assessment, the pitfalls of having…
Wednesday May 15, 2024