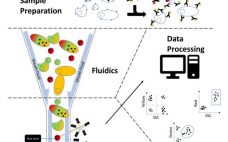
This webcast features: Jamie Hall, Project Manager, Center of Excellence for Biopharmaceuticals, Intertek Pharmaceutical Services Flow cytometry is a powerful technique which can be applied to all stages of the drug development pipeline including discovery, clinical development, CMC, regulatory submission and in support of production. It allows the analysis of complex mixtures of particles, from polystyrene beads to cells. Utilizing a broad spectrum of fluorescent detectors, assays can be set up to assess multiple parameters at once and ascertain otherwise…
Wednesday, August 7, 2019 Daily Archives
FDA and Novartis back Zolgensma amid data manipulation issues
The US FDA remains confident that Zolgensma will not be pulled after being alerted to allegations of data manipulation by the gene therapy manufacturer AveXis. In May 2019, Novartis company AveXis received US Food and Drug Administration (FDA) approval for its $2.1 million single-dose, one-time gene therapy Zolgensma (onasemnogene abeparvovec) to treat children less than two years of age with spinal muscular atrophy (SMA). A month later, AveXis informed the Agency “about a data manipulation issue that impacts the accuracy…