Ajinomoto Bio-Pharma Services is a fully integrated contract development and manufacturing organization (CDMO), with sites in Wetteren and Balen (Belgium) and San Diego (California, USA). We provide comprehensive process development services, current good manufacturing practice (CGMP) manufacturing, and drug-product fill–finish services of small-molecule and biologic-active pharmaceutical ingredients (APIs) and intermediates. Fill out the form below to read this capabilities review now.
Friday, August 2, 2019 Daily Archives
26 Years of Biologics Development and Manufacturing Experience
Avid Bioservices is a full-service, dedicated contract development and manufacturing organization (CDMO) focused on development and manufacturing of biopharmaceutical products derived from mammalian cell culture. Avid provides process development and current good manufacturing process (CGMP) clinical and commercial product manufacturing and offers expertise supporting analytical development, qualification, and validation activities. Additional service offerings include cell line optimization, cell culture and feed optimization, product characterization, and stability testing. Avid has 26 years of experience producing a comprehensive range of proteins, including…
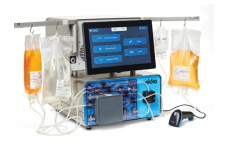
ekko™ Acoustic Cell Processing System
The ekko™ system is a good manufacturing practice (GMP)–capable platform technology for cell and gene therapy manufacturing. Based on the innovative application of acoustics, the ekko system can process cells gently and efficiently in ways that traditional mechanical and filtration methods cannot. With a wide range of operating conditions, the ekko system can be used to concentrate and wash cellular material in multiple unit operations, from early research through to GMP production. Fill out the form below to read the…
A booster shot for vaccines: Univercells working to cut production costs
Vaccines cost too much to make according to Univercells, which aims to have a novel platform ready for commercial-scale production by 2020. Vaccines have had a dramatic impact on human health. One recent study argued that vaccine development “represents humankind’s most important and successful endeavor… over the last 200 years” [1]. Many diseases that caused millions of deaths in generations past have been stopped in their tracks by industry-supported vaccination programmes run by entities like World Health Organization (WHO), UNICEF and…
Freezing of T Cells Using the VIA™ Freeze System
Autologous T-cell therapies no longer are limited to small, single-site trials. National and international demand is growing, bringing with it the demand for rapid scaling of manufacturing. But there is little margin for error, because any variables in an end-product could result in a potentially hazardous change to a therapy’s potency. Cryopreservation is a critical tool for quality control and transport of cells. Liquid nitrogen (LN2)–based controlled-rate freezers have long been used to freeze clinical-grade T cells. Cells are frozen…
Process for Production of Oncolytic Adenovirus
Oncolytic viruses constitute a new promising therapeutic approach for treating cancer. These viruses selectively replicate in tumor cells and effectively kill those cells without harming normal cells. Selectively engineered, the viruses do not only destruct target tumor cells, but they also stimulate the host’s antitumor immune response. Adenoviruses are extensively characterized, well-studied viral vectors that infect both dividing and nondividing cells without the risk of integration into the host genome. Generally causing a mild nature of disease, adenoviruses are considered…
The Next Milestone in Cell and Gene Therapy
The field of cell and gene therapy is transforming the way patients who are diagnosed with cancers or genetic diseases can be treated. Today, the cost of producing these therapies still represents a major hurdle on the way to the commercialization. New technologies are needed that enable robust and cost-efficient manufacturing and yield replicable high-quality medicines. Although the therapeutic opportunities for patients are exciting, the stakes for patients and drug developers are high. We want to be your partner, add…
Rentschler Biopharma: Excellence Driving Us Forward
Rentschler Biopharma SE, a leading contract development and manufacturing organization (CDMO), has been at the forefront of biologic development and manufacturing for over 40 years. Our value chain encompasses the whole process: from gene to vial and from concept to market. As a full-service provider, Rentschler Biopharma offers its clients one primary contact, with only one contract signed for all phases of a development cycle. As a provider of high-end solutions for our partners, we help clients transition a project…
GE: Learning from MAb automation to tackle CAR-T
GE Healthcare says monoclonal antibodies laid the groundwork for its workflow technologies, allowing accelerated uptake of automation in the autologous cell and gene therapy space. In May, bioprocessing tools and services firm GE Healthcare launched its Chronicle automation software for cell therapy manufacturing. The offering supports the complete workflow for cell therapy products including the optimization of manufacturing from process development to commercialization. Talking to Bioprocess Insider at the BPI Theater at BIO in June, Catarina Flyborg, GM of GE’s…