Quality by design (QbD), risk management, and new technologies are shaping biologics formulation work in the 21st century. We saw much evidence of this at the BioProcess International Conference and Exhibition in Boston last fall, where a wide range of talks filled the Drug Product, FillâFinish, and Formulations track during the week after Labor Day. Dingjiang Liu (Regeneron) offered a high-level discussion from the BioPhorum Development Group (BPDG) on âAn Intercompany Perspective on Biopharmaceutical Product Robustness Studies.â Such studies ensure…
Wednesday, March 20, 2019 Daily Archives
Analytical Strategies for Fixed-Dose Coformulated Protein Therapeutics
Coformulation of two or more proteins in a single formulation is an emerging approach to delivering multiple biotherapeutics that previously have been administered in sequence. This approach brings multiple benefits to all stakeholders. Foremost for patients, the primary benefits are combined therapeutic effects and improved convenience (e.g., fewer administration events). Healthcare providers see logistical benefits and decreased risk of medical errors. Additionally, coformulations also simplify manufacturing logistics, reduce costs of packaging and distribution, and provide new opportunities for product portfolio…
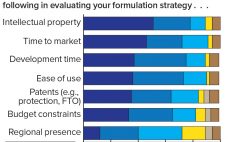
Early Stage Development of Advanced Formulations in the Drug Development Process Provides Competitive Advantages: Survey Predicts That Drug Product Formulation Recognition and Budgets Will Increase Significantly
New antibody formats and aggregate-prone, subcutaneously administered protein therapeutics present biopharmaceutical companies with major challenges regarding protein stability and aggregation. At the same time, protein stability often is not given enough attention in early stages of development. Protein aggregation reduces drug activity so that increasing doses are needed to achieve the same desired effect. Even worse, protein aggregates can induce immunogenecity that endangers patients and compromises product approval. A market study presented for the first time at the Bio-Europe 2018…
From 1mL-100L: Consistent Chromatography Performance Across Multiple Sizes of Pre-Packed Columns
This webcast features: Fletcher Malcom, Product Line Leader, Repligen With the increasing use of pre-packed columns in protein separations, it is critical to demonstrate consistent chromatographic performance at different scales. This allows for seamless process scale-up and scale-down. This webinar will show how chromatography separation remains unchanged across different OPUSÂź Column sizes. Performance results obtained with bench-scale columns (â„1mL column volume) are demonstrated to be scalable to production sized columns (â€100L column volume). Watch the recorded webcast below.
BioProcess International Europe 2019: Event Planner
BioProcess International Europe has earned its reputation as the leading and largest European bioprocessing industry event. It is a key time each year when the industry connects to share new ideas and innovations across all phases of bioprocessing: from cell line development through upstream and downstream, with bioprocess analytics, viral safety, continuous manufacturing, and vaccines. This yearâs meeting on 2â4 April 2019 at the Messe Wien Exhibition Congress Center in Vienna, Austria will attract more than 900 scientists, engineers, and…
Genentech on tech transfer: âSmall volumes are all a bit new to usâ
Genentech says it is looking to disruptive technologies for a fast and agile response to capacity demands as its portfolio evolves away from large volume biologics. At BPI West in Santa Clara last week, Eric Fallon, senior director of Pharma Technical Innovation of Technology & MSAT at Genentech, spoke about the difficulties in driving performance and carrying out tech transfers within its large global manufacturing network. âOur manufacturing infrastructure is built around large, economies of scale, facilities. They can be…
Access to smart money a key life science growth driver, Dynamk
Bioprocess executives moving to the financial sector shows a strong desire for continued investment in the life sciences space, says Daniella Kranjac from VC firm Dynamk. Reinhard Vogt joined venture capital (VC) firm Dynamk Capital as a new general partner and managing director earlier this year, following an illustrious career in bioprocessing. Bioprocess Insider recently spoke with Vogt about major investment opportunities, but in this follow-up Q&A, Daniella Kranjac, co-founder and managing partner at Dynamk, explains how his expertise strengthens…