Tracking Antibodies in the Clinic
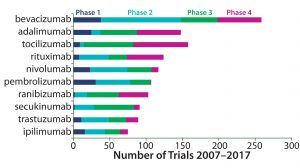 In tracking clinical trial activity around the world, GlobalData has identified 5,273 clinical trials of monoclonal antibodies (MAbs) that started between 1 January 2007 and 31 December 2016. The top three drugs in numbers of trials were bevacizumab, adalimumab, and tocilizumab. Rounding out the top 10 were rituximab, nivolumab, pembrolizumab, ranibizumab, secukinumab, trastuzumab, and ipilimumab.
In tracking clinical trial activity around the world, GlobalData has identified 5,273 clinical trials of monoclonal antibodies (MAbs) that started between 1 January 2007 and 31 December 2016. The top three drugs in numbers of trials were bevacizumab, adalimumab, and tocilizumab. Rounding out the top 10 were rituximab, nivolumab, pembrolizumab, ranibizumab, secukinumab, trastuzumab, and ipilimumab.
Healthcare analyst Marco Borria said, “All the top 10 drugs are marketed, and the majority of trials initiated for them were phase 3 trials, closely followed by phase 4 and phase 2.” But the largest share of bevacizumab trials were in phase 2, and 35% of all studies covered indications for which no drugs have yet been approved.
ISCT Supports FDA Regen-Med Policy
Late in fall 2017, the International Society for Cellular Therapy (ISCT) offered its support for the newly released comprehensive regenerative medicine policy framework from the Food and Drug Administration (FDA). ICT’s >1,500 cell therapy experts from >50 countries share a vision to translate cellular products into safe and effective therapies to improve patients’ lives around the world.
The new framework clarifies the agency’s risk-based regulations for drugs, devices, and biological products in regenerative medicine, including processes for maintaining safety and efficacy. It also describes fast-track approval processes for new therapies. An important component explains enforcement actions that will be taken against companies promoting unproven therapies, treatments, and products that raise potential safety and ethical concerns.
ISCT leaders support continued clarification and action from the FDA on regulating the cell therapy industry. The group has worked for years to develop initiatives for dealing with potentially harmful, unproven cellular therapies (www.celltherapysociety.org/page/UCT). It advocates stronger legislative oversight and improved frameworks for regulating unproven products and clinics. As regulatory agencies worldwide review these FDA actions, ISCT leadership particularly welcomes the agency’s strong stance.
FDA qualifications for fast approval of gene and cell therapies that prove efficacy and sustained therapeutic benefit also will provide considerable incentive to the sector. Together with actions from other regulators, this new framework will support the international regenerative medicine industry, ultimately speeding delivery of therapies to patients.
“ISCT welcomes FDA efforts to provide additional regulatory clarification for all those operating in the cell therapy field,” said ISCT president Catherine Bollard. She urges caution, however, in the agency’s introduction and application of this framework. “The FDA needs to balance bringing those operating outside the regulatory pathways to compliance, taking action against those that remain outside the licensing and regulatory frameworks, and continuing to foster the ongoing innovation and considerable potential for the majority of the sector operating within the regulatory frameworks.”
Over-zealous regulatory action, Bollard warns, could increase development and manufacturing bureaucracy and costs — and ultimately time to market by delaying validation of products and facilities. “Ultimately, though, patients are the customers of cell and gene therapies. They have a fundamental right to expect a scientific and clinical rationale for a proposed treatment.” Bollard says ISCT is working to resolve issues such as development cost, pricing, and reimbursement.”
Stand Up to Cancer Catalyst Launches 10 Clinical Trial Projects
The Stand Up to Cancer (SU2C) charity established its Catalyst program last spring to accelerate research on cancer prevention, detection, and treatment. Charter supporters are Merck, Bristol-Myers Squibb, and Genentech (Roche). As of fall 2017, SU2C Catalyst had launched 10 clinical trial projects combining cancer treatments from nine different pharmaceutical companies. The focus is to study combined treatments with multiple medicines, devices, and therapies as well as standard-of-care treatments. These projects will test 16 FDA-approved immunotherapy compounds in new combinations for new indications and in combination with preoperative radiation therapies as well as epigenetic and DNA repair therapies — with support from the American Association for Cancer Research.
The program brings together researchers who collaborate across academic and corporate boundaries on clinical trials covering a range of cancers to deepen our understanding of why (not just whether) treatments are effective. These inaugural projects will explore new uses for a number of powerful medicines, both new compounds and approved agents that can be investigated for other uses. Medicines and grants to support the trials are provided to the charter supporters as well as AbbVie, Astex Pharmaceuticals, Iovance Biotherapeutics, Mirati Therapeutics, Prometheus Laboratories, and TESARO.
SU2C President and CEO Sung Poblete said, “This unique industry/academic collaboration has reduced the time to get clinical trials started by over 75% while bringing significant scientific rigor to the selection and oversight of the projects. SU2C actively manages these grants to minimize delays and support the achievement of key milestones.”
These combination treatments (in some cases triple combinations) were proposed by the research community responding to competitive requests for proposals. Those were evaluated and selected by industry-specific subcommittees predominantly composed of leading academic scientists. The 10 inaugural studies address a range of cancers.
“Companies often focus on an approval pathway for a specific indication,” said Phillip Sharp (chairman of the SU2C scientific advisory committee, institute professor at Massachusetts Institute of Technology, and Nobel laureate). “SU2C Catalyst broadens the approach by asking the scientific community how best to use a treatment in any setting and in any combination. These projects promote combinations that would not likely be considered without this program even before they’re approved for other indications.”
Despite the astounding potential of immunotherapy, researchers don’t yet know why it works only for certain people. SU2C Catalyst projects are aimed at learning how to prime people to respond and how to make that response last. Both positive and negative results will drive breakthroughs for the best outcomes for the most patients.
Progress for DNA Origami
DNA’s double-stranded structure gives it strength. Using a technique known as “DNA origami,” biophysicist Hendrik Dietz has been building nanometer-scale biomolecular objects at the Technical University of Munich (TUM) for several years. In 2017, he and his team broke out of the nanometer realm to build larger molecular objects while cutting the costs of doing so by a thousand-fold. These innovations open a new frontier for an emerging technology.
Dietz’s team has transferred viral construction principles to DNA origami technology, making it possible to design and build structures on the scale of viruses and cell organelles. The technology builds on a long single strand of nucleic acids that is appended to a double-stranded structure using short staple sequences. The double-stranded structure is sufficiently stable energetically that researchers can force the single strand into a desired shape using appropriately chosen counterparts, explains Dietz.
 Building Polyhedral Structures: By adding side groups, his team can modify and insert chemical functionalities into the resulting objects. Based on their previous experience in the nanometer realm, the team can build larger structures using prefabricated parts. They first created V-shaped nanometer-sized objects with shape-complementary binding sites that allow those objects to autonomously attach to each other while floating in solution. Depending on the opening angles of those Vs, they form “gears” with a controlled number of spokes. The team next created new building blocks that allow those “nanogears” to form long tubes.
Building Polyhedral Structures: By adding side groups, his team can modify and insert chemical functionalities into the resulting objects. Based on their previous experience in the nanometer realm, the team can build larger structures using prefabricated parts. They first created V-shaped nanometer-sized objects with shape-complementary binding sites that allow those objects to autonomously attach to each other while floating in solution. Depending on the opening angles of those Vs, they form “gears” with a controlled number of spokes. The team next created new building blocks that allow those “nanogears” to form long tubes.
“At lengths of one micrometer and a diameter of several hundred nanometers, these tubes have reached the size of some bacteria,” Dietz says. “We can use the architecture of individual elements to determine features of the overall structure.”
His team then constructed new elements that should assemble into self-limiting cage structures. Again depending on the V angle, a defined number of units merge to form tetrahedral, hexahedral, or dodecahedral structures in a second step. These cages attain molecular weights and sizes comparable to those of viruses and small cell organelles. “A potential future application of artificial cages is transport of medication in the body,” Dietz suggests. The goal would be to release active agents only at specific target locations.
Large-Scale Production: Early manufacturing processes could produce only a few micrograms of these structures at a time. Chemical production of the short staple DNA strands (base by base) was the bottleneck. Obtaining a main strand from bacteriophages enables large-scale biomanufacturing. The team has refined so-called synthetic DNA “enzymes” (strands that break apart at specific positions when exposed to a high concentration of zinc ions) for joining the short staple sequences to a long strand.
“Once precisely assembled with a specific base sequence,” Dietz explains, “these combined strands can be reproduced in a biotechnological process, as with single strands of bacteriophage DNA.” Combined strands have been produced successfully using high-density bacterial culture. The process is scalable and thus amenable to high-volume production on a large scale. Increasing the zinc-ion concentration after DNA isolation releases the short staple sequences, which then fold the main strands into desired shapes. TUM scientists have made multiple grams of four different DNA-origami objects and say that scaling up the process to cubic meters is within their grasp.
“The interplay of biotechnology and process technology has enabled setting a truly fundamental milestone on the path to future applications in DNA nanotechnology,” says Dirk Weuster-Botz, chair of the Institute of Biochemical Engineering, a group that has collaborated with the Dietz team.
