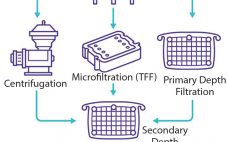
Clarification is typically the first unit operation in the purification of monoclonal antibodies (MAbs) and other proteins from mammalian cell cultures. This process removes cells and cellular debris from the culture fluid to produce a clarified cell culture supernatant that will be suitable for further purification (1). Before 2000, depth and tangential-flow microfiltration were standard clarification technologies in the biopharmaceutical industry. Process-scale centrifugation was considered to be a significant capital investment, and bioprocessors had limited ability to control (minimizing) the…
Thursday, April 19, 2018 Daily Archives
Filter-Based Clarification of Viral Vaccines and Vectors
Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with…
Ensuring the Integrity of Single-Use Containers: Providing Robustness, Science, and Helium-Based Technology with a Detection Limit of 2 ÎĽm
Identifying the greatest defect size, both for liquid leaks and microbial ingress, is a fundamental step toward protecting the integrity of single-use systems (SUS) under real process conditions. Integrity testing of such systems may become a prerequisite in the future because they are used in the most critical process steps, with detection limits correlating to liquid leaks and microbial ingress. Such testing guarantees the sterility of drug substances and drug products packaged in single-use systems and, therefore, enhance patient safety.…
Your Brand Is the Patient’s Experience
The future success of biopharmaceutical businesses will depend at least partly on their ability to create meaningful brand experiences from the start of a drug program. By “brand,” I don’t mean logos and taglines. I’m talking about meaningfully unique experiences that directly affect clinical and patient needs — specifically, to address the growing demand for self-administered injectable therapeutics. Whether you are a biosimilar developer trying to carve out differentiated value or a market leader looking at your patent protection in…
Scaling Up? No Problem with New 80cm Diameter Pre-packed OPUS Columns
This webcast features: Fletcher Malcom, Director of Product Management, Downstream Technologies, Repligen The technical and economic benefits of ready-to-use, pre-packed chromatography columns have been proven and documented. The question remains, how large can we go? This presentation will show how the unique design of the new 80cm pre-packed OPUS column delivers chromatographic performance, and how usage can result in substantial cost and time savings for clinical and commercial scale manufacturing.
Ask the Expert: Integration of Cell Line, Process, and Analytical Technologies: Speeding Development and Clinical Supply of Emerging Therapy Products
Chinese hamster ovary (CHO) cells are being pushed to their productivity limits in drug development and biomanufacturing. Biopharmaceutical companies working on promising new therapies increasingly struggle with development challenges such as weak protein expression, low-yield purification steps, and poorly optimized analytical techniques. To address those challenges, innovators need robust cell-expression platforms and advanced process, analytical, and biomanufacturing technologies. Since 2012, KBI Biopharma has performed development and/or manufacturing services using more than 20 different cell lines generated by Selexis SA. In…