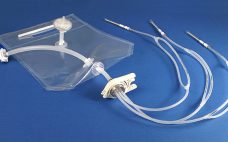
New sterile molded filling assemblies from AdvantaPure continue the shift to single-use processing methods that save time, reduce cross contamination risks and increase productivity between batches of biopharmaceutical and pharmaceutical products. “These assemblies were developed specifically for vial and syringe filling,” notes Lawrence Morano, AdvantaPure’s global sales manager. “Our team of engineers is well versed in single-use, biopharmaceutical manufacturing, and fluid flow, and we’re able to work closely with customers to design systems that meet their individual process requirements.” Manufactured…
Tuesday, December 19, 2017 Daily Archives
U.S. Approval of Three Rapid Microbiological Methods for MACI Product Release
Short time frames are a major challenge in developing alternative microbiological methods for autologous cell therapy products. Ideally, results are made available in under a day. Obtaining regulatory acceptance also can be a challenge, but it is made easier if methods are included in an application (e.g., a biologics license application, BLA) rather than changing a method that is already part of an approved process. Comparing different detection platforms can be a challenge if they have different readouts, and validation…
Comparing Adherent-Cell Technologies: Amplification of Virus Stocks and Viral Vectors
FUJIFILM Diosynth Biotechnology (FDB) is a multifunctional commercial development manufacturing organization (CDMO) that manufactures virus-based therapeutics. Its site in Texas specializes in both adherent and suspension cell culture for production of virus vectors and for development of analytical assays to support production and expression of our clients’ therapeutics. We work with a number of cell lines such as human embryonic kidney (HEK293) and human retinal cells (Johnson & Johnson/Crucell’s proprietary PER.C6 line), which can grow in suspension. However, many of…