GEA Service recently launched its new VISIOCOVER™ Upgrade Program for the safe visual inspection of non-sticky-powder processes in the dairy, food, chemical and pharma industries. For customers currently using SANICOVER™ to monitor their powder flow, fluidization and cleaning, the upgrade provides three standard sizes to match the SANICOVER™ systems. Installing the VISIOCOVER™ on existing SANICOVER™ installations is easy and allows the technician to safely inspect the production without opening the cover, which would risk contaminating the product. The VISIOCOVER™ Cam…
Monday, December 11, 2017 Daily Archives
Control of Single-Use System Supply Chains
Single-use technologies are now dominant for the clinical production of biopharmaceuticals and are becoming more mainstream within commercial manufacturing facilities. They allow biologics manufacturers to decrease the footprint of their facilities by approximately 20% because of a reduced need for utilities that generate water, steam, and clean-in-place solutions. Engineers believe that the capital outlay for a single-use facility is 25–45% less than for a facility based on stainless steel equipment. Similarly, they estimate that such facilities need half the water…
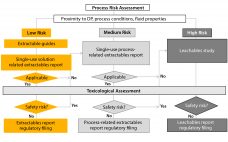
Exploring the Science Behind Single-Use Container–Closure Integrity Assurance
Failures in the integrity of single-use systems during commercial manufacturing can cause a number of serious problems for biomanufacturers. A loss of system integrity during processing can allow environmental contaminants that can be dangerous for patients (e.g., microbes) to enter a process. Biopharmaceuticals and their intermediates can be highly potent or even infectious agents, so an integrity failure can jeopardize the safety of operators. In severe cases when biomanufacturers cannot ensure the quality of drug products for fear of a…
Single-Use Production Platforms for Biomanufacturing
The pipeline of biopharmaceuticals remains strong, and the market for biologics could exceed US$450 billion by 2025. Analysts predict that sales within segments such as regenerative medicine and antibody–drug conjugates (ADCs) will grow faster than 20% each year. Yet considerable challenges remain for biopharmaceutical companies to overcome if they wish to be successful. First, they must reach the market quickly. Analysis by the Boston Consulting Group shows that the proportion of available value that a newly launched product can capture…
Control of Critical Process Parameters Using In-Situ Analytics
Biopharmaceutical companies can develop entire end-to-end single-use production platforms and use them for commercial manufacture of their biological products. Single-use facilities are flexible, can be implemented quickly, and do not require the large up-front capital investments needed for stainless steel equivalents. However, single-use facilities must be supplied with a large quantity of high-quality consumables. Biomanufacturers should pay close attention to their supply chains for those consumables to ensure that they are robust, integral, and fully compatible with biological expression systems…
Prescriptive Analytics for Bioprocessing Platforms
The preceding articles have shown how biopharmaceutical companies increasingly are adopting single-use manufacturing technologies for commercial production facilities as their confidence grows in the material science, supply chains, and robustness of single-use systems. Single-use suppliers can support adoption of end-to-end process platforms in commercial manufacturing settings with dedicated teams of experts in process development, engineering, and regulatory support. Advances in process analytical technology (PAT) are providing engineers with greater information on conditions within their single-use bioprocess platforms to allow for…
Final Thoughts: Single-Use Platforms
The development and launch of new biopharmaceutical products is a very challenging and risky process. Sponsors must get their products into clinical testing as quickly as possible to beat their competition and take the greatest share of the market. Yet that need for speed must not lead to companies launching products with inefficient manufacturing processes that ultimately will leave them vulnerable to attack from low-cost competition. Single-use systems have been a significant enabling technology for companies developing new biologics because…