Sartorius Stedim Biotech (SSB), a leading international supplier for the biopharmaceutical industry, announced the launch of its next generation BIOSTAT STR®, a fully scalable, single-use bioreactor family based on a conventional stirred-tank design. This new bioreactor range featuring upgraded hardware and software, as well as a fully integrated, new design of Flexsafe STR® single-use bags, ensures quick and easy bioprocess scale-up of biologics and vaccines. The BIOSTAT STR® bioreactors are equipped with an improved stainless steel bag holder for user…
Thursday, May 4, 2017 Daily Archives
Charter Medical Announces Exclusive Global Distribution Agreement with INCELL Corporation
Charter Medical Ltd., a Fenner PLC subsidiary (LSE:FENR), a globally recognized manufacturer of products for the regenerative medicine and bioprocessing industries, announced today that it has established a strategic partnership with INCELL Corporation, an innovative developer and manufacturer of specialty medias and formulated solutions for tissue and cell collection, transport, processing and storage. The partnership will enable both organizations to meet the growing customer demand for more comprehensive solutions in cell culture, cell expansion, and cryopreservation. Mary Pat Moyer, Founder,…
Sartorius Stedim Biotech Launches Chemistry Testing Services
Sartorius Stedim Biotech (SSB) announced the launch of a full range of chemistry testing services. Offered by its subsidiary Sartorius Stedim BioOutsource, they characterize the physicochemical properties and structural attributes of therapeutic monoclonal antibodies (MAbs). The service platform methods have been developed to ensure rapid sample analysis and reporting for MAbs and biosimilars. This comprehensive range of chemistry testing services complies with the well-established ICH Q6B scientific guidelines for pharmaceuticals for human use, and includes methods to characterize protein structure,…
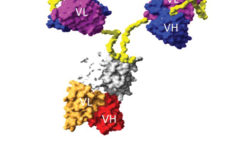
Solving Problems in the Production of Complex Proteins and Other Biologics
This webcast features: James Brady, PhD, VP of Technical Applications and Customer Support, MaxCyte, Inc. Moving bispecific and complex protein therapeutics from the bench to the clinic requires a scalable process that is consistent and reproducible while delivering a biologically active product. MaxCyte’s delivery platform is a high performance, electroporation-based technology that can rapidly generate milligram to gram quantities of protein in the cell line of choice. You will learn: How to express quality bi-specific antibodies, bi-specific T cell engaging…