This webcast features: Tim Schroeder, Director, Product Management at Repligen GmbH Formerly Atoll pre-packed columns, OPUS® pre-packed chromatography columns for process development are used in all steps of downstream development, including screening, process validation and viral clearance, scale up and sample preparation. This webcast will demonstrate how OPUS® ValiChrom Columns, glass pre-packed columns that are exact scale-down models of corresponding full-scale chromatography columns, are ideal for process validation including viral clearance. It will also demonstrate how OPUS® RoboColumns®, miniaturized columns…
Friday, January 20, 2017 Daily Archives
Downstream Disposables: The Latest Single-Use Solutions for Downstream Processing
Downstream processing has been considered a “bottleneck” in the manufacture of protein biotherapeutics ever since cell culture engineers began dramatically improving production efficiencies around the turn of the century. And as single-use technologies have grown in importance and acceptance, offering more solutions every year, their biggest challenges too have been in the separation, purification, and processing that follows product expression in cell culture. Many of the technologies familiar to process engineers — e.g., centrifugation and chromatography — present technical and…
Single-Use Depth Filters: Application in Clarifying Industrial Cell Cultures
For current process development phases, many biomanufacturers’ attention is directed increasingly to the first unit operation in downstream processing, which is the removal of cells and cell debris from culture broth and clarification of supernatant containing a biopharmaceutical product. Given the high cell densities achievable with both mammalian and microbial cell culture processes, primary recovery can be a significant challenge. The current trend in cell culture is to increase product titers with enriched culture media, improved cell productivity, and increased…
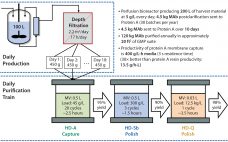
Multicolumn Chromatography: Facilitating the Commercialization of Monoclonal Antibodies
Since 2001, global contract development and manufacturing organization (CDMO) CMC Biologics has completed more than 120 projects with at least 100 pharmaceutical partners. During that time, the company has taken a holistic approach to helping clients balance manufacturing risks and rewards. The team focuses on evaluating key technologies to deploy a constantly evolving set of capabilities in support of biopharmaceutical clients throughout their product lifecycles. Part of that commitment is continually evaluating what would best benefit customers and where key…
Membrane Adsorbers, Columns: Single-Use Alternatives to Resin Chromatography
Filtration membranes are used extensively throughout the biopharmaceutical industry for a range of applications, from coarse filtration to nanofiltration. Advantages of filter technologies include easy scaling, disposability, and (for many membrane filters) rapid and robust performance in a single-pass. The same advantages have been realized with membrane adsorbers. Chromatography resins are inherently disadvantaged by diffusion limits of the pores in chromatography media. Therefore, resin columns must be significantly oversized to match the performance of high productivity bioreactors. By comparison, membrane…
Single-Use Fill and Finish: An Interview with NNE Pharmaplan
I talked with NNE Pharmaplan’s Kim Vincent Andersen (single-use technology and biotechnology specialist) and Niels Guldager (global technology partner in biotech) to discuss their experiences with client facilities that incorporate significant elements of single-use technology. In particular, they highlighted a recent project for Novo Nordisk involving a large-scale greenfield filling and inspection facility in Hillerød, Denmark. Find more detailed information about the project online at https://goo.gl/yp4LQh. And you can watch a video about it here: https://youtu.be/czwwgdt3CxI. A Case Study You…
From the Editor for January
Happy New Year from all of us at BPI. As you can see on the cover, 2017 is the start of our 15th year of publication. Launching a magazine is an uncertain prospect in any era, especially within the trade press format, and our addition of peer-review gives us a split personality. But we are not immune to the print-versus-digital conundrums facing publishers these days, and an anniversary year provides a logical platform on which to update editorial approaches. I…
Spotlight for January
New Bioanalytical Testing Laboratory In November 2016, Sartorius Stedim Biotech (SSB) opened a new bioanalytical and biosafety testing laboratory in the biotech hub area of Boston, MA. This dedicated laboratory is designed to accommodate rising North American demand for the company’s BioOutsource specialized assay platforms and facilitate ongoing expansion of products and testing services. The company performs a range of testing for biologics, vaccines, and biosimilars throughout their development and manufacture. Reinhardt Vogt is a member of SSB’s executive committee.…