From the Editor Many publishers are grappling with managing and predicting the course of print and digital publishing. For academic journals with relatively few paid subscribers and/or association members focusing on specialty subject matter, going digital may make good sense — especially if that can lower subscription costs. However, BPI is a hybrid of reviewed journal and trade publication, addressing many reading and advertising preferences. The choice of which content to offer in print and which to duplicate or offer…
Tuesday, January 13, 2015 Daily Archives
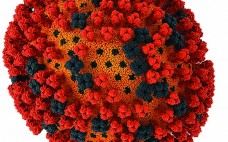
Fundamental Strategies for Viral Clearance – Part 1: Exploring the Regulatory Implications
Over the past several decades, biologics such as monoclonal antibodies (MAbs) and recombinant proteins have provided therapeutic benefits and efficacy for the treatment of human disease. Completion of the human genome project (launched in 1990) produced a draft of the genome in 2001. A full sequence was published on the 50th anniversary (2003) of the initial publication of Watson and Crick’s papers on the double-helical structure of DNA (1). That large volume of genetic information has been translated into usable…
Advances in Chromatography Automation
Not long ago, chromatography automation meant strip recorders and peristaltic pumps. Today, few people would consider that to be true automation, and even fewer would settle for binders full of strip-recorder paper reels. Automation is becoming intelligent and in the process is making our workflows smarter. But how close is automation to being as smart as an experienced scientist? Bio-Rad Laboratories spoke with academics, biotechnology R&D scientists, and industrial process engineers about the evolution of chromatography automation — where it…
The Single-Use Watering Hole: Where Innovation Needs Harmonization, Collaboration, and Standardization
Within the past few years, the single-use technology (SUT) arena of the biopharmaceutical industry has exploded in growth. Leading organizations have predictably and understandably stampeded to the “watering hole” of single-use to drink up the advantages that disposable components offer over traditional multiuse parts and technologies. The initial value and risk-reduction results are being realized — but not without the emergence of other trade-offs. End users continue to call for standardization in emerging areas of the industry while also recognizing…
The CMC Strategy Forum Series, Part 1 – QbD and Risk Management
Introduction by Cheryl Scott The CMC Strategy Forum series provides a venue for biopharmaceutical product discussion. The meetings focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of the forum meetings are published in BioProcess International. This process is meant to help ensure that biopharmaceutical products manufactured with…
Perfusion’s Role in Maintenance of High-Density T-Cell Cultures
T-cell therapy is a rapidly growing field of personalized medicine, attracting the interest of venture capitalists and pharmaceutical companies alike. Such therapies exploit T cells’ innate abilities to protect against pathogens as well as to seek and destroy cancerous cells. Although many different forms of T-cell therapies are currently in clinical trials, they all follow a common protocol: T cells are isolated from a patient, modified and expanded in a laboratory setting, and then infused back into the same patient…
Bioreactor Design for Adherent Cell Culture — The Bolt-On Bioreactor Project, Part 1: Volumetric Productivity
The Bolt-on Bioreactor (BoB) project is an independent initiative aimed at developing and commercializing a bioreactor for efficient, automated culture of adherent cells in production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges. The first challenge has to do with volumetric productivity, the second with process automation, the third with containment and sterility, and…
Evaluating Freeze–Thaw Processes in Biopharmaceutical Development – Small-Scale Study Designs
Regulations mandate that biopharmaceutical product quality be controlled throughout manufacturing, storage, transportation, and delivery to patients (1). Operations often include freezing and thawing of a bulk drug substance, dilution of that purified substance to a target concentration, filtration, filling into a selected container–closure system, additional processing (e.g., lyophilization), inspection, packaging, storage, transport, and delivery (2). Freezing is a common processing step used to maintain stability and quality of a drug substance during development and production of biopharmaceutical products. It is…
Recruiting and Market Share Reshape Life Sciences Facilities and Locations
Where a biopharmaceutical company does business affects its profitability — as does how it manages its facilities and even where its non–customer-facing operations are located. In fact, facilities and real estate represent some of the biggest expenses for such companies. And yet, they are often overlooked. In today’s shifting global life-sciences landscape, site selection and management strategies are coming to the forefront as companies seek operating efficiencies, access to multidisciplinary talent, and cost-effective facilities. The following insights from JLL’s 2014…