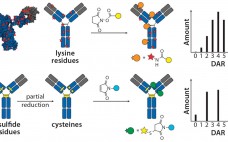
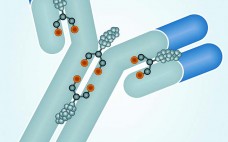
Monoclonal antibodies have dominated the biopharmaceutical market for over two decades. Few people doubt that their future success in fields such as oncology, inflammation, and autoimmune diseases owes much to the development of antibodies conjugated to cytotoxic drugs. Like all great innovations, this is a breathtakingly simple concept: Combine the targeting specificity of an antibody with a small-molecule drug as an effector component joined to that antibody by means of a small chemical or peptide linker, and you have a…
Wednesday, October 15, 2014 Daily Archives
Bioprocessing Challenges of Antibody–Drug Conjugates
Development of highly potent active pharmaceutical ingredients (HPAPIs) is clearly a pharmaceutical industry trend. Highly potent drug products involve active agents and APIs that are so potent therapeutically (or simply just outright toxic) even in small dose that special precautions are required during their manufacture — particularly when handling the active agents. Such requirements include maximal containment and isolation of the process stream. Worker exposure and environmental release clearly pose problems. The necessity and intensity of containment efforts with HPAPIs…
The Next Step in Homogenous Bioconjugate Development: Optimizing Payload Placement and Conjugate Composition
[Audio Recording] Bringing a new biologic drug to market is a long and expensive process, with research and development (R&D) cycles that can span up to 15 years and may cost over a billion dollars. Biologic drug development also involves significantly more complex manufacturing and CMC components than does development of small molecules. Nonetheless, the pharmaceutical industry is increasingly shifting its R&D efforts to focus on biologic drugs. According to a recent report from Tufts Center for Study of Drug…
Bioconjugation Reaction Engineering and Kinetics Simulation
Bioconjugates represent an important and growing class of pharmaceuticals that include PEGylated proteins, vaccines, and antibody-drug conjugates (ADCs) (1–8). Numerous protein conjugation techniques exist (9). Among the more important conjugation chemistries used for protein therapeutics are N-hydroxysuccinimide (NHS), aldehyde, and maleimide (10–13). To date, process development of industrial biopharmaceutical conjugation reactions has largely been empirical in nature. Typically, many experiments testing different reaction parameters are required to identify optimal process conditions. In some instances, nonmechanistic statistical models can be used,…
Fine-Tuning ADCs for Best-in-Class Therapeutics
Antibody–drug conjugates (ADCs) use the targeting ability of a monoclonal antibody (MAb) to deliver a highly biologically active drug to diseased cells while sparing healthy cells, creating potent and effective therapies. This emerging class of novel drugs currently focuses almost exclusively on cancer treatment. Two blockbuster ADCs — brentuximab vedotin (Adcetris from Seattle Genetics) for treatment of rare lymphomas and ado-trastuzumab emtansine (Kadcyla from Genentech/ Roche, manufactured by Lonza) for treatment of HER2-positive metastatic breast cancer — have improved treatment…
Predicting Aggregation Propensity and Monitoring Aggregates in ADCs
Antibody–drug conjugates (ADCs) are monoclonal antibodies coupled to cytotoxic agents with stable linkers. ADCs travel to target cells, where the antibody binds to its antigen expressed on the cell surface. Upon binding, the full ADC can be internalized by a process called receptor-mediated endocytosis. That process is followed by lysosomal degradation of ADC complexes, which ultimately leads to release of the cytotoxic agent and apoptosis of the target cell. Drugs used in ADCs can be up to a thousand times…
Technology Advances Enable Creation of Better ADCs
Antibody–drug conjugates (ADCs) for treatment of cancer combine the tumor-targeting properties of antibodies with the cell-killing properties of cytotoxic drugs. By targeting a drug to a tumor, it is possible to reduce systemic toxicity and thereby enable administration of drugs that are otherwise too toxic to be effective therapies. Although the concept of an ADC is simple, in reality developing an effective treatment is somewhat more challenging. Whether an ADC has sufficient efficacy at a tolerable dose depends on four…
Using a CMO for Your ADC: Access Analytical and Manufacturing Platforms, Specialized Facilities, and Expertise
[Audio Recording] Antibody–drug conjugates (ADCs) are an exciting new area of therapeutics. They bring the “magic bullet” that was promised by Paul Ehrlich over a hundred years ago to reality by targeting cancer cells to deliver chemotherapies without poisoning a patient’s whole body. ADCs offer a promising form of therapy by providing higher safety margins than traditional chemotherapeutics alone, and they make selectivity possible. We should be able to personalize a therapy to the specific cancer expressed in a given…
Your Shake Flask is not a Black Box Anymore! Online Measurements of Metabolic Activity Accelerate Your Bioprocess Development
This webcast features: Dr. Gernot Thomas John, director of marketing and innovation at PreSens Precision Sensing GmbH Shake Flasks are widely used for bioprocess development. The most widely measured parameter to determine the biomass is the optical density OD. Typically, this parameter is measured not continuously and offline. Now PreSens is launching the new SFR vario. This compact device is placed underneath the shake flasks and measures 4 parameters online: pH, Oxygen, Oxygen consumption (OUR) and biomass. Join GT as…
Promethera Biosciences Receives Approval in Belgium to Enroll Patients in the Imminent HEP002 Phase 2b/3 Clinical Trial
Promethera Biosciences, a Belgian biotechnology company developing Promethera® HepaStem, a cell-based therapy for the treatment of both orphan liver-based metabolic diseases and acquired liver diseases, today announces that Belgium has authorized the conduct of a trial designed to treat paediatric patients with urea cycle disorders (UCD) in a Phase IIb/III study (HEP002). This first agreement officially opens the start of the second clinical trial conducted by the company. Belgian patients will be recruited and treated in Cliniques Universitaires Saint-Luc in…