Product Development
Service: Contract development and manufacturing
Applications: Clinical- and commercial-scale complex biologics
Features: Therapure Biopharma specializes in biologics development, scale-up, and manufacturing. At its 130,000-ft2 CGMP facility in Toronto, Ontario, Canada, the company offers cell-line, process, and analytical development; product manufacturing and testing; and aseptic fill–finish and lyophilization. It has >20 years of experience working with mammalian and primary cell cultures, whole blood, and plasma sources.
Contact Therapure Biopharma www.therapurebio.com
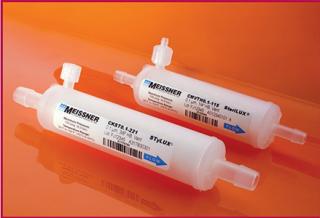
Capsule Filters
Product: CM and CK capsule filters
Applications: Small-volume, single-use biocontainers and tubing assemblies
Features: Meissner’s CM and CK capsule filters are designed for integration into single-use systems. Their compact size minimizes fluid hold-up volume. These capsules can be ordered with no vents, one vent, or with dual vents in either Luer ports or sanitary vent valves. They come with the full range of Meissner membranes or can be specified with a range of PP microfiber and glass-fiber media. Gamma irradiation ensures product sterility.
Contact Meissner Filtration Products, Inc. www.meissner.com
Shaker Incubator
Product: Multitron Pro
Applications: Small-scale cell culture
Features: This successor to the Multitron 2 incubation shaker has an enhanced user interface with a modern, intuitive touch controller and a number of configuration options. A double-glass door expands the workable temperature range and improved performance with a humidifier. Further options include cooling, illumination, and an accessory for disposable-bag cultivation.
Contact Infors AG www.infors-ht.com
Therapeutic Cell Culture
Product: BD Mosaic hMSC culture medium, supplement, and culture surface coating
Applications: Mesenchymal stem-cell therapies and research
Features: BD Mosaic serum-free medium provides a complete cell culture environment for human mesenchymal stem cells derived from bone marrow. It is designed to shorten the time for cell expansion to about half that of other serum-free media, achieving two or more doublings per passage (without refeeding) with a low minimum seeding density of 3,000–4,000 cells/cm2. This is the first generation of BD’s line of full serum-free “environments” for stem-cell expansion, which includes both supplements and cell culture surface coatings as well as the culture medium.
Contact BD Biosciences www.bd.com
Gene Expression
Product: GS Xceed gene expression system
Applications: Recombinant protein production through animal cell culture
Features: The GS Xceed system provides up to a six-week reduction in cell line construction time for fast generation of clonal cell lines. This platform is the latest generation of Lonza’s platform for titers of up to 6 g/L. The GS system has been used to create >100 high-producing cell lines and 13 marketed biologics. Companies worldwide can access the new system through a research evaluation agreement or commercial license. Key to the new system is the CHOK1SV GS knock-out cell line, in which both alleles of the endogenous glutamine synthetase gene have been knocked out.
Contact Lonza Group Ltd. www.lonza.com/gsxceed
Contamination Control
Product: Abbott PLEX-ID system
Applications: Detection and identification of microbial contamination in biomanufacturing processes
Features: The PLEX-ID system is the only high-throughput, molecular-based technology for rapid, broad detection and identification of thousands of virus, bacteria, or fungi direct from samples without the need for culturing single agents or mixtures down to the strain level. The system comes stand-alone or as part of a complete solution that includes sample extraction and processing equipment for routine operation. It uses high-resolution mass spectrometry to determine microbial base composition across multiple loci. Unique molecular signatures identify single or multiple organisms at trace levels in a range of matrices.
Contact Ibis Biosciences www.ibisbiosciences.com/biopharma
Culture Media Analysis
Service: Spedia-Predict service
Applications: Production process development and optimization
Features: The Spedia-Predict service monitors and validates process-critical parameters for managing culture media consistency in production environments. The approach rapidly profiles most constituents in a medium, delivers quantitative data, and can be applied equally to fully chemically defined or complex media. Markers are identified for correlation with bioprocess performance and monitor batch consistency. This helps prevent failure and performance variability in large-scale production, enabling efficient and cost-effective processes.
Contact Spinnovation Biologics www.spinnovation-biologics.com
Fluid Management
Product: Polysulfone stopcocks
Applications: Fluid control with single-use tubing assemblies
Features: Qosina has added one-way, three-way, and four-way polysulfone stopcocks to its line of disposable fluid-control components made in an ISO 9001/14001 facility. White handles clearly indicate open and closed positions. Polysulfone is more chemically resistant than most plastics. The one-way low-profile model has a female Luer-lock inlet and rotating male spin-lock outlet. The three-way model has two female Luer locks and a male Luer slip (with three caps). And the four-way model features a radiation-grade tint with two female Luer locks and a male Luer slip.
Contact Qosina www.qosina.com
Process Control
Product: BioPAT MFCS/win software
Applications: Bioprocess automation
Features: This SCADA system for both reusable and single-use bioprocess applications can trigger different actions automatically based on events. For reproducible and fully automated batch processing, the program mirrors each step with customized recipes that comply with the ANSI/ISA-88.01 standard for batch control. Semi- or fully automatic operations, state or time-dependent transitions enable organized and structured batch processing as well as flexible manufacturing. Linked Umetrics MODDE DoE software aids in process optimization.
Contact Sartorius Stedim Biotech www.sartorius-stedim.com/en/products/process-control-tools-software/biopat-mfcs
Single-Use Bioreactor
Product: Mobius CellReady 50-L stirred-tank bioreactor
Applications: Process development and pilot-scale mammalian cell culture
Features: The unique rigid base and top-panel design of Mobius CellReady bioreactors enables simple, reliable, and robust installation. Mobius SensorReady technology provides for flexibility in monitoring and controlling processes using a range of conventional and disposable sensor technologies with a standardized bioreactor process container. Open architecture allows flexibility in the choice of automation platforms. With this addition, the company now offers a range of disposable bioreactors from 3-L to 200-L scales. A turn-key system includes Finesse automation; a modular system can be integrated with different platforms.
Contact EMD Millipore www.millipore.com/mobius
Transient Transfection
Product: PEIpro transfection reagent
Applications: Protein, antibody, and viral vector production
Features: This next-generation linear polyethylenimine (PEI) transfection reagent extends the Polyplus line for large-scale transient transfection. It was tailored to meet current regulatory guidelines for raw materials used in bioprocessing — as an alternative to lipid-based technologies. This animal-product–free option is optimized for platforms such as suspension-adapted mammalian cell lines cultivated in shaker flasks, platform shakers, or stirred-tank bioreactors. A fully GMP-compliant version is available on request. Quality control testing includes a transfection efficiency test to ensure lot consistency.
Contact Polyplus-transfection SA www.polyplus-transfection.com
Anticounterfeiting
Product: Track-and-trace solutions
Applications: Pharmaceutical packaging and logistics
Features: Recently acquired by Mettler-Toledo, Pharmacontrol Electronic offers track and trace technologies in Europe, the United States, and Asia. Products include the Datamatrix XMV, advanced bundling, shipping, manual aggregation and bottle serialization stations, and Pilot Line and Pilot Site Manager software. Modular and standardized systems mark all units as they move through production: single cartons, bottles, and vials; bundles; shipping cases and palettes. PCE systems can be scaled up to meet changes in legal requirements and are easily integrated into manufacturing operations.
Contact Pharmacontrol Electronic GmbH www.pharmcontrol.de