Packed-bed basket technology developed by New Brunswick Scientific (acquired by Eppendorf Inc. in 2007) provides a shear-free environment for production of animal cells. Because little information is available on the utility of the New Brunswick CelliGen BLU single-use bioreactor system for production of secreted proteins (especially in perfusion mode), this study was conducted to measure growth and productivity of alkaline phosphatase (ALKP)–secreting rCHO cells. We used two packed-bed bioreactor types — a 5-L New Brunswick CelliGen BLU single-use vessel and a 2.5-L autoclavable glass vessel — both operated by New Brunswick CelliGen 310 console in perfusion mode. Perfusion provides a homeostatic environment for optimal cell growth similar to that experienced by cells in vivo, where waste products are constantly removed and fresh nutrients are replenished. Cells cultured in packed-bed bioreactors are not exposed to hydrodynamic forces, which allows for maximum cell growth and protein expression (1). Our objective was to determine whether any differences are observed between the productivity of the two bioreactors.
Results and Discussion
Glucose Utilization and Lactate Production: Glucose is the main energy source for cell proliferation and ALKP production. So we expected glucose levels to directly correlate with ALKP production in each experiment. Because lactate is a secondary energy source, its levels were expected to decline following this initial increase and the use of glucose in the media. Lactate metabolism is beneficial to the system by reducing a major metabolic by-product from the system (2, 3). Glucose levels measured at the time of induction (day 3) were nearly 0 g/L in both experiments. Media lactate concentrations increased in response to decreasing glucose availability. The use of lactate as a secondary energy source can also be observed as lactate levels decrease at each 2-L perfusion.
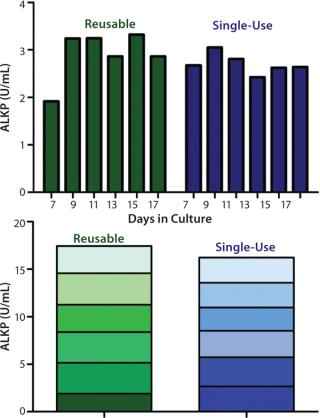
Comparison of Bioreactor Systems for ALKP Production:Figure 1 shows average total ALKP production per trial. Overall, there is no significant difference in ALKP production between the two bioreactor systems. The total amount of ALKP measured after five media exchanges in the reusable vessel was 17.44 U/mL, 16.22 U/mL in the single-use vessel.
In summary, our results show no significant differences in ALKP production between these packed-bed bioreactor systems operated in perfusion mode. Given the greater productivity of cells cultured in a packed-bed bioreactor and its many advantages operated in perfusion mode, researchers scaling up mammalian cell culture for protein production should strongly consider using the New Brunswick CelliGen® BLU packed-bed, single-use bioreactor system.
Author Details
Taylor Hatton, Shaun Barnett, and Kamal Rashid (associate director of the Center for Integrated BioSystems) are at Utah State University, 4700 Old Mani Hill, Logan, UT 84322; kamal.rashid@usu.edu. This application note study was partially funded by Eppendorf Inc. For full app note visit www.eppendorfna.com.
REFERENCES