The development of insect cell culture production systems has produced opportunities for the expression of recombinant proteins for research and therapeutic applications in a non–mammalian cell culture environment. Various insect cell lines have been developed for the baculovirus expression vector system (BEVS). Development of stable transfected Drosophila and lepidopteran cell lines has offered a nonlytic culture for the production of recombinant proteins from insect cells.
Spodoptera frugiperda Sf9 cells have been successfully adapted to suspension culture and have become an attractive platform for industrial-scale cell culture, lending them for use in stirred-tank bioreactors, including single-use bioreactors (S.U.B.s). S.U.B. vessels reduce capital costs, cleaning, maintenance, and installation and validation time in comparison with traditional, stainless-steel vessels. The Thermo Scientific HyClone S.U.B. can be used to culture cells at scales of 25 to 2,000 Lin working volume. Such bioreactors use the familiar and proven stirred-tank technology typical of traditional stainless-steel bioreactors but in a disposable format. This allows the same support hardware to be used for different processes with rapid turnaround, without the risk of cross contamination and eliminating the need for CIP/SIP and validation otherwise required.
The study described here details the use of the Thermo Scientific HyClone S.U.B. in culturing Sf9 cells using an animal-derived-component–free (ADCF), serum-free, and protein-free medium (Thermo Scientific Hyclone SFM4Insect) and feed supplements for high-density insect cell culture. Materials and Methods
Cells and Culture Medium: Sf9 cells were adapted to HyClone SFM4Insect (Thermo Fisher Scientific, SH30913), a serum-free, protein-free and ADCF medium developed to support baculovirus. Recombinant protein production was used as the basal medium. HyClone Insect Feeds (IF1-5) and Cell Boost 3 (Thermo Fisher Scientific, SH30825) were used at various time points (see Process and Feeding Schedule and Analytics below) throughout the culture.
S.U.B. Operating Parameters: Cells were scaled-up in SFM4Insect to a 30-Lworking volume in a Thermo Scientific HyClone 50-LS.U.B. with dual sparge (a microsparge and macrosparge integrated into one bioreactor bag) system.
The culture parameters used were
- Temperature = 27 °C
- Agitation rate = 150 rpm
- pH 6.4, not actively controlled
- Dissolved oxygen (O2) = 50%
- Sparge outlay:
- macrosparger (open pipe) air flow rate = 30 LPM
- microsparger (frit) air flow rate = 2.0 LPM;
- microsparger (frit) O2 flow rate ≤2.0 LPM as needed
- Seed density = 0.75×106 cells/mL.
Process and Feeding Schedule and Analytics: Samples were taken using the needleless sample port on the S.U.B. bag at least every 24 hours to determine cell population density (CPD), viability, and metabolite profile. Cell counts, viabilities, and average cell diameters were determined using a Beckman Coulter ViCell. Metabolite and pH concentrations were determined using a Nova Biomedical Bioprofile FLEX, running samples at a dilution 1:5. Offline metabolite concentrations were monitored by diluting the sample at a 1:5 dilution, followed by analysis using Nova Biomedical Bioprofile FLEX.
Cells were cultivated in a fed batch mode, based on the following feed regime:
- Feed 1 (after cells have reached 4×106 viable cells/mL): 15% IF3.1, 1% IF2.1, 0.5% IF1.1
- Feed 2 (after cells have reached 8×106 viable cells/mL): 15% IF3.1, 1% IF2.1, 0.5% IF1.1
- Feed 3 (after cells have reached 13–16×106 viable cells/mL): 15% IF3.1, 1% IF 2.1, 0.5% IF1.1, 1% tyrosine stock, 1% cysteine stock, 1% IF5.1, 0.1% HCl to neutralize solutions
- Glutamine and IF4.1 (carbohydrates) were to be added as needed based on analysis from the Bioprofile FLEX
- 3% simethicone antifoam emulsion was used as needed to control foaming.
Results and Discussion
Culture Evaluation: The S.U.B. was charged with 30 Lof SFM4Insect and allowed to reach the temperature setpoint of 27 °C. The pH probe was calibrated before autoclaving, and the DO probe was calibrated once it was attached to the S.U.B. During the calibration process, 2 mL of antifoam (mix with 7 mL of ADCF SFX-Insect) was added to control foaming. After calibration was completed and process control stabilized at set points, the bioreactor was seeded with 0.75×106 cells/mL. Feeding of the culture was completed as below:
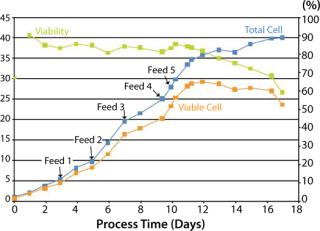
- Feed 1: (day 3, viable cell density: 4.57×106/mL): 15% IF3.1, 1% IF2.1, 0.5% IF1.1
- Feed 2: (day 5, viable cell density: 8.31×106/mL): 15% IF3.1, 1% IF2.1, 0.5% IF1.1
- Feed 3: (day 7, viable cell density: 14.3×106/mL): 15% IF3.1, 1% IF 2.1, 0.5% IF1.1, 1% Tyrosine stock, 1% Cysteine stock, 1% IF5.1, and 0.1% HCl to neutralize solutions
- Feeds 4 and 5: Glutamine was fed on days 9 (350 mL) and 10 (1 L), and IF4.1 was fed on day 9 (1% or 500 mL).
Supernatant samples that had been collected throughout the study were analyzed using HPLC for amino acid content. Cells reached a maximum viable cell population density of ~28×106 cells/mL (Figure 1) with viability >80% from seeding through peak density. Results showed that the feed schedule maintained sufficient levels of most amino acids. However, it cysteine and tyrosine were low at times and could be used in additional feeds in future optimization runs.
Conclusions
Spodoptera frugiperda Sf9 cells were successfully cultured to high density in animal-derived-component–free (ADCF) medium (SFM4Insect) and feeds (IF1-5) using a single-use, stirred-tank bioreactor. Some potential limiting factors (cysteine and tyrosine) with the present feeding regime were noted, leaving room for further optimization work.
Author Details
Cory Card is director of process development; Bill Whitford is senior marketing manager; and Paula Decaria is a scientist at Thermo Fisher Scientific, 925 West 1800 South, Logan, UT 84321;1- 435-792-8000; http://www.thermoscientific.com/hyclone.