Vaccine engineering, antibody quality screening, and glycosylation profiling processes can generate large quantities of protein samples that must be analyzed in a timely manner. Generation of high-quality data and rapid, quantitative analyses are mandatory to determine next steps. Although still heavily relied upon, sodium dodecyle sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is labor-intensive and only semiquantitative, and its throughput capacity is limited. Quantitative analysis techniques such as capillary electrophoresis (CE) and high-performance liquid chromatography (HPLC) do not provide the throughput necessary to move processes forward efficiently. The Caliper Life Sciences’ LabChip GXII system delivers quantitative, high-throughput protein analysis necessary to truly accelerate these development work flows. LabChip Protein Analysis
The automated LCGXII system performs rapid, quantitative protein analysis. Samples are aspirated from microplates using a single-sipper microfluidic chip. Staining, destaining, electrophoretic separation, and fluorescent detection take place on a protein LabChip device, eliminating the need for SDS-PAGE analysis. In addition, LabChip HT software presents relative concentration, molecular weight, and percent purity results for each protein automatically. Data are available for immediate review in virtual gel, electropherogram, and table summary formats. These capabilities make the LCGXII ideal for vaccine development and quality control, antibody quality assessment, and N-glycan profiling.
LCGXII Protein Assay Features
Process samples directly from 96-and 384-well plates. Assays are complete in just 40 seconds. DNA and RNA assays also available.
• Protein sizing: 14–200 kDa
• 70×faster than traditional CE
• Ideal for crude lysate analysis
• 21 CFR Part 11 compliant software
LCGXII Vaccine Applications
• Characterization of viral DNA, RNA, proteins and virus-like particles (VLPs)
• Viral preparation purification monitoring
• Subunit and VLP stability and degradation
• Glycoform macro and micro heterogenicity
• Rapid typing and subtyping of viral genotypes
• Viral titer determination
• Viral protein purity and integrity
Vaccine Development and QC
Increased global anxiety over pandemic threats and adequate vaccine supplies have fueled the ongoing need for more efficient vaccine development and production. In recent years, technologies that provide more detailed analytical data have played a key role in improving the vaccine engineering process. LabChip technology has been widely adopted within vaccine development and QC processes, where it is used for a variety of applications including viral strain typing, subunit expression optimization and tracking, characterization of virus-like particles (VLPs), and quality control testing of vaccine purity. The high-throughput, quantitative analysis provided by the LCGXII system easily integrates into existing workflows, and early studies have shown that use of LabChip technology could reduce the typical five-month vaccine production process by up to four weeks.Table 1: Protein assay results for different plasmids and proteins at scale
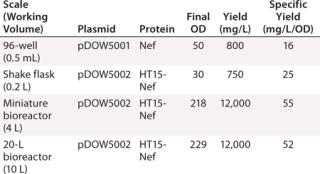
Subunit Vaccine Expression Monitoring: Researchers at Dow Pfenex monitored expression levels of a subunit vaccine in Pseudomonas fluorescens at different fermentation scales, from microplates to a 20-L reactor (Figure 1). They were able to rapidly identify the pathways to increase product yields and reported a 10-fold increase in yield production when using the LabChip protein assay for high-throughput expression monitoring.
LCGXII Antibody Screening Applications
Determination of critical quality attributes for protein therapeutics such as titer, purity, and the following:
• Degree of glycosylation
• Covalent aggregates
• QC of single-chain Fvs
• Purification process development Fragmentation and Ab assembly
• Purification development
• Clone selection
• Cell culture optimization

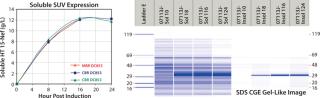
Antibody Quality Screening
Quality by design (QbD) implementation requires high-throughput experimentation to identify and thoroughly understand the relationship between critical process parameters and a product’s critical quality attributes. The resulting design of experiment (DoE) studies produce large numbers of samples that can greatly exceed the capacity of analytical laboratories using HPLC- and CE-based separations to monitor protein product quality. These high-throughput demands are easily addressed using the LCGXII system, which delivers quantitative results 70×faster than traditional methods. In addition, the LabChip protein assay also provides the analytical precision, dynamic range, and resolution required when determining sample purity.
Monoclonal Antibody Analysis — LabChip Assay Comparison to Capillary Electrophoresis: The authors of a recent Amgen publication on monoclonal antibody screening concluded that the LabChip protein assay provides comparable resolution and sensitivity but on a time scale that is ~70×faster (41 seconds compared with 50 minutes per sample) than conventional CE separation (1). Figure 2 shows actual run-time data.

High Throughput N-Glycan Profiling
Glycans N-linked to the Asn-X site in the Fc region of recombinant monoclonal antibodies (rMAbs) have been shown to affect pharmacokinetics, efficacy, and therapeutic safety (2). Operational parameters in antibody manufacturing — such as media, pH, and temperature — can affect these glycosylation patterns. Glycosylation profiling is required to accurately characterize therapeutic antibodies. The traditional chromatography, CE-LIF, and mass spectrometry workflows used for N-glycan analysis can take up to three days for digestion, labeling and analysis. This lengthy process can be reduced to less than six hours using the new LabChip N-glycan profiling assay for the LCGXII system.
LabChip N-Glycan Assay Comparison to NPLC and CE-LIF: A Pfizer study comparing the LabChip N-glycan assay to traditional NPLC and CE-LIF analysis found that LabChip analysis is 160×faster than NPLC and 6×faster than CE-LIF analysis with comparable results as shown in Figure 3.
LabChip N-Glycan Profiling Assay Features
• Reagents provided in a convenient 96-well plate format
• Streamlined protocols (less than six hours from preparation to final results)
• One-hour deglycosylation
• CE-LIF process takes place on-chip
• Samples are analyzed in just 60 seconds
• Identification of main glycans
• Enzymatic digestion
• No reductive amination

• Degree of glycosylation
• Covalent aggregates
• QC of single-chain Fvs
• Purification process development Fragmentation and Ab assembly
• Purification development
• Clone selection
• Cell culture optimization


Antibody Quality Screening
Quality by design (QbD) implementation requires high-throughput experimentation to identify and thoroughly understand the relationship between critical process parameters and a product’s critical quality attributes. The resulting design of experiment (DoE) studies produce large numbers of samples that can greatly exceed the capacity of analytical laboratories using HPLC- and CE-based separations to monitor protein product quality. These high-throughput demands are easily addressed using the LCGXII system, which delivers quantitative results 70×faster than traditional methods. In addition, the LabChip protein assay also provides the analytical precision, dynamic range, and resolution required when determining sample purity.
Monoclonal Antibody Analysis — LabChip Assay Comparison to Capillary Electrophoresis: The authors of a recent Amgen publication on monoclonal antibody screening concluded that the LabChip protein assay provides comparable resolution and sensitivity but on a time scale that is ~70×faster (41 seconds compared with 50 minutes per sample) than conventional CE separation (1). Figure 2 shows actual run-time data.

High Throughput N-Glycan Profiling
Glycans N-linked to the Asn-X site in the Fc region of recombinant monoclonal antibodies (rMAbs) have been shown to affect pharmacokinetics, efficacy, and therapeutic safety (2). Operational parameters in antibody manufacturing — such as media, pH, and temperature — can affect these glycosylation patterns. Glycosylation profiling is required to accurately characterize therapeutic antibodies. The traditional chromatography, CE-LIF, and mass spectrometry workflows used for N-glycan analysis can take up to three days for digestion, labeling and analysis. This lengthy process can be reduced to less than six hours using the new LabChip N-glycan profiling assay for the LCGXII system.
LabChip N-Glycan Assay Comparison to NPLC and CE-LIF: A Pfizer study comparing the LabChip N-glycan assay to traditional NPLC and CE-LIF analysis found that LabChip analysis is 160×faster than NPLC and 6×faster than CE-LIF analysis with comparable results as shown in Figure 3.
LabChip N-Glycan Profiling Assay Features
• Reagents provided in a convenient 96-well plate format
• Streamlined protocols (less than six hours from preparation to final results)
• One-hour deglycosylation
• CE-LIF process takes place on-chip
• Samples are analyzed in just 60 seconds
• Identification of main glycans
• Enzymatic digestion
• No reductive amination


High Throughput N-Glycan Profiling
Glycans N-linked to the Asn-X site in the Fc region of recombinant monoclonal antibodies (rMAbs) have been shown to affect pharmacokinetics, efficacy, and therapeutic safety (2). Operational parameters in antibody manufacturing — such as media, pH, and temperature — can affect these glycosylation patterns. Glycosylation profiling is required to accurately characterize therapeutic antibodies. The traditional chromatography, CE-LIF, and mass spectrometry workflows used for N-glycan analysis can take up to three days for digestion, labeling and analysis. This lengthy process can be reduced to less than six hours using the new LabChip N-glycan profiling assay for the LCGXII system.
LabChip N-Glycan Assay Comparison to NPLC and CE-LIF: A Pfizer study comparing the LabChip N-glycan assay to traditional NPLC and CE-LIF analysis found that LabChip analysis is 160×faster than NPLC and 6×faster than CE-LIF analysis with comparable results as shown in Figure 3.
LabChip N-Glycan Profiling Assay Features
• Reagents provided in a convenient 96-well plate format
• Streamlined protocols (less than six hours from preparation to final results)
• One-hour deglycosylation
• CE-LIF process takes place on-chip
• Samples are analyzed in just 60 seconds
• Identification of main glycans
• Enzymatic digestion
• No reductive amination
Author Details
Richard P. Bunch is microfluidics product manager at Caliper Life Sciences, 68 Elm Street, Hopkinton, MA 01748; Rick.Bunch@caliperls.com. All rights reserved. Caliper, the Caliper logo, and LabChip are tradenames and/or trademarks of Caliper Life Sciences, Inc. All other names are trademarks of their respective companies.
REFERENCES
1.) Chen, X. 2008. Microchip Assays for Screening Monoclonal Antibody Protein Quality. Electrophoresis 29:4993-5002.
2.) Jefferis, R. 2005. Glycosylation of Recombinant Antibody Therapeutics. Biotechnol. Prog. 21:11-16.
