Process Analytical Technology (PAT)–based in-line buffer dilution (IBD™) systems produce either binary or ternary blends with tight adaptive control. Up to 100× concentrates are diluted to meet the final dilution end product (1×) specifications. The conductivity of the diluted end product can be controlled to within ±0.1% of the upper limit of the user-defined conductivity range of the detector, and to ±0.1 pH units. Two experiments that were designed and performed to illustrate this blending accuracy are discussed below.
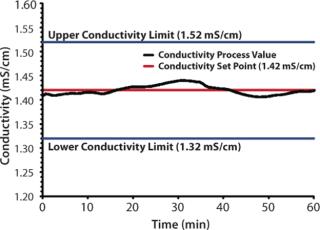
Experiment 1In the first experiment (Figure 1), a standard preparation of 10 mM sodium phosphate buffer, pH 6.7, was created using a 5 L/min IBD™ skid. The product was prepared by blending a 10× concentrate (100 mM) of sodium phosphate, pH 6.7, with deionized water using patented adaptive PAT feedback control. For this run, the span of the conductivity detector was set from 0 to 100 mS/cm, which allowed for a specification limit of ±0.1 mS/cm. The conductivity set point for the 10× dilution was determined by recording the conductivity (1.42 mS/cm) of a carefully prepared standard of the end product (10 mM Sodium Phosphate, pH 6.7). An optimization run was first performed to equilibrate the system and determine initial start speeds. Next, in the actual “product run,” the pumps were ramped-up to predetermined optimized start speeds for 15 seconds, at which point the system transitioned into automatic mode where the conductivity of the buffer was maintained by adaptive PAT control to within specification for one hour (Figure 1).
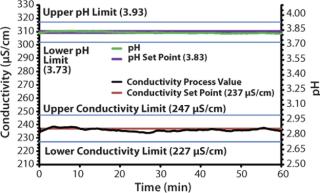
Experiment 2 illustrates the ability of the IBD™ system to produce a ternary blend while adaptively controlling conductivity and pH simultaneously. In addition, an extremely dilute Tris-Acetate buffer (2.5 mM Tris, 27.5 mM acetate, pH 3.83) was chosen to illustrate the ability of the IBD™ system to control conductivity accurately within the S/cm range. In this experiment, deionized water, 100 mM acetic acid, and 100 mM Tris-base were fed into the inlets of pumps A, B, and C, respectively. The conductivity and pH set points were determined as in experiment 1. The range of the conductivity detector was set from 0 to 1,000 S/cm, which allowed conductivity control to within ±10 S/cm. Conductivity and pH set points were 237 S/cm and pH 3.83.
As before, once the optimized start speeds timed out, automatic mode took control and both conductivity and pH were maintained within specification for 1 hour using PAT feedback control (Figure 2).
Process scale buffers can be produced using adaptive PAT-based in-line-buffer-dilution to meet high accuracy requirements for sensitive bioprocess applications. This technology is ideally suited for both every day, simple buffers used in bioprocess applications, and for complex ternary blends.