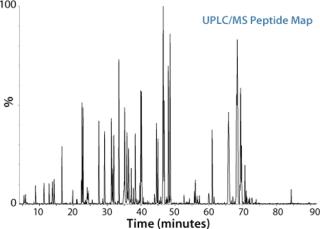
Peptide mapping is the workhorse technique in biopharmaceutical analysis, offering the comprehensive characterization of biopharmaceutical products. Its applications include the identification of proteins based on the elution pattern of peptide fragments, the determination of posttranslational modifications, the confirmation of genetic stability, and the analysis of protein sequences when interfaced to a mass spectrometer.
In a typical peptide mapping workflow, a protein is first digested with trypsin or other protease to generate peptides, which are separated by liquid chromatography, most commonly reversed-phase LC, and then detected by either UV/VIS or MS. Compared with a traditional LC-UV/VIS approach, LC/MS analysis significantly enhances the information content available from a peptide mapping experiment. Measurement by MS can differentiate coeluting peptides under a chromatographic peak, and can identify and locate low-abundance modifications and single amino acid variants of a protein by MS/MS.
Although LC/MS peptide mapping can be powerful in detecting and identifying minute changes in a protein therapeutic, many organizations struggle to develop a robust and reproducible analytical workflow. Poor sample preparation, faulty chromatographic methodology, low resolution, inaccurate measurement, and time-consuming manual data annotation all can diminish the utility of this technique.
Based on a thorough understanding of LC/MS peptide mapping experimentation, Waters’ previously-described analytical approach can be applied as a highly robust and efficient workflow to vastly improve the results of peptide mapping studies. The peptide mapping workflow includes RapiGest™ SF assisted proteolytic digestion, high resolution separation of peptides with the ACQUITY UPLC® System, a multiplexed MSE approach for accurate mass measurement of all the eluting peptides as well as corresponding fragments with Xevo™ QTof MS, and automated data processing and interpretation by BiopharmaLynx.
The attributes of each and all these techniques translate into increased user confidence in results that are achieved faster than possible with traditional methods. The solutions are simple, straightforward, and the results are coherent and comprehensive. The ability to quickly, routinely, and comprehensively characterize biopharmaceutical products will improve and accelerate the drug development process, ensure the quality of biopharmaceuticals, and protect intellectual property.
For the full application note, and other biopharmaceutical applications from Waters, download our application book or order a copy by mail at www.waters.com/xevoapps.