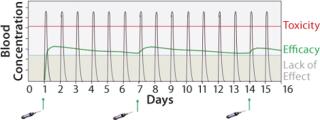
Biological drugs are an increasingly important and growing part of the pharmaceutical industry but are associated with a number of challenges that have constrained their application. The lack of efficacy due to short protein half-life and/or poor availability is an issue that leads to a requirement for both high and frequent dosing (Figure 1). Such administration demands can result in unwanted side effects and limit the therapeutic benefits, as well as elevated patient dosing that creates an extra burden on manufacturing capacity and treatment costs. Albumin fusion technology (albufuse) has been designed to address these shortcomings.
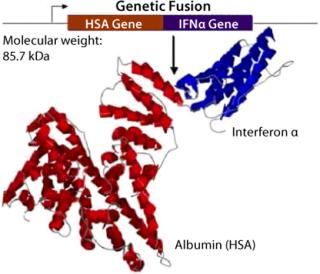
Albumin is a benign natural scaffold and carrier protein found at high concentrations in plasma with a typical half-life of 20 days. albufuse takes advantage of these properties of albumin; it is the molecular fusion of albumin to protein drug candidates providing a single hybrid protein from a contiguous cDNA for the “target” peptide or protein with DNA encoding albumin. The technology is a single-step expression solution that does not involve the additional postmanufacture chemical processing and purification steps required by other technologies, such as PEGylation for extending circulatory half-life. Simplifying the production of proteins helps manufacturers achieve significant cost savings. This pioneering method makes it possible to produce completely new therapeutics with a naturally longer in vivo half-life. Research has demonstrated that albumin fusion molecules can be effectively transported around the body, thus reducing the risk of localized retention at the site of administration.
The albufuse technology can be used in conjunction with an extended range of protein expression systems from yeast to animal cells. Novozymes Biopharma employs a yeast expression platform that is highly capable of producing albumin fusion molecules in an efficient manner. Based on a 2-µm plasmid construct, the system allows stable expression of an extensive range of proteins, including protease inhibitors, enzymes, transport proteins, cytokines, anti-angiogenic polypeptides, anti-inflammatory polypeptides and growth hormones (Figure 2). The main advantage of this protein expression system is the ability to generate intact proteins at a relatively low cost because of large-scale manufacturing in conjunction with lower purification costs. The Novozymes yeast expression system is free of animal-derived components, a matter of growing importance. A further key benefit of the system is that it provides a highly consistent and reliable supply of the therapeutic protein of interest.
Albumin fusion technology is applicable to a great number of peptides and proteins. The heart-shaped albumin molecule has several points, particularly at either the C- or N- termini, where proteins and peptides can be placed. It is even possible to fuse two different proteins together with albumin in the same recombinant molecule to provide two different functions (bivalent) at the same time. As a result, the albufuse concept can be used to produce novel bifunctional proteins while also helping to improve existing products.
Antibody Fragmentsalbufuse technology is also suitable for antibody fragment expression applications. To date, the use of antibody-based therapeutics has been limited by the high costs of process development and commercial-scale manufacture. In response, Novozymes Biopharma’s scientists focused on achieving high-level expression of scFv albumin protein fusions of >5 g/L.
For additional information about licensing Novozymes Biopharma’s albufuse or yeast expression technology, please refer to the contact below.
B
FOR DEVELOPERS
-
Superior drug candidates over existing drug or competitor products
-
Enhanced stability during manufacturing and in final formulation
-
New IPR opportunities around development of novel molecules
FOR MANUFACTURERS
-
No postproduction or conjugation losses
-
Increased yields of poorly expressed proteins
-
Specific matrices available for optimal downstream purification
-
Potential for favorable cost of goods
-
Proven at cGMP and manufacturing scale
FOR PATIENTS
-
Less frequent administration
-
Improved efficacy: drug maintained at optimum levels
-
Reduced side effects
-
Greater tolerance
-
Improved compliance: increased likelihood of positive clinical outcome