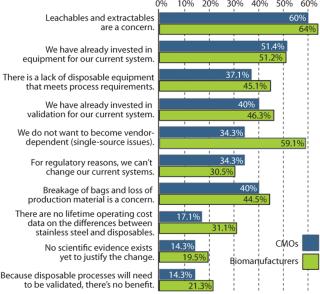
In biopharmaceutical manufacturing, single-use components and systems can offer distinct advantages over reusable, cleanable systems. Deciding whether to move to a single-use approach, however, depends on many factors. In a recent review of biomanufacturers and CMOs, the risk of leachable materials entering drug products was highest on a list of end-user concerns, as shown in Figure 1 (1). That’s not surprising in view of the high organic polymer content of disposable components, a general inexperience with such polymeric materials in process contact, and the need to ensure that safety and regulatory requirements are satisfied.
For example, the US FDA’s GMP requirements for finished pharmaceuticals states, “Equipment shall be constructed so that surfaces that contact components, in-process materials, or drug products shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements” (2). The task of assessing leachable materials in process fluids may appear to be very difficult and demanding, especially for multicomponent polymeric systems containing flexible containers, tubing, filter capsules, connectors, and so on. Furthermore, individual components may be supplied by different vendors and also may have diverse materials of construction.
Detailed information on extractable or leachable compounds, however, can be discovered through the use of sophisticated analytical equipment. In addition, recent industry recommendations have been issued on disposable manufacturing systems to facilitate validation. For example, the Bio-Process Systems Alliance (BPSA) offered detailed recommendations to assist end-users in risk assessment of specific products and process systems as well as testing programs (3, 4).

WWW.PALL.COM
Those recommendations define clearly the distinction between extractables testing and leachables testing. Extractables are compounds that can migrate from a material into a model solvent under exaggerated test conditions. Leachables are compounds that are shown to migrate into a drug product in final-dosage containers under normal process conditions. Their quantities are typically a relatively small proportion of total extractables. Extractables studies can show the potential for a material to release leachables into a drug product or process fluid. Such data provide an important platform on which decisions can be made regarding the need for or extent of leachable substances testing in process fluids or final drug formulations and dosage containers. Furthermore, because extractables data are usually generic and involve model solvents, they can be made available by suppliers to end users for incorporation into users’ own test program assessments and regulatory submissions.
This first installment of a series on components and single-use systems describes supplier-generated extractables testing of two individual components typically used in integrated single-use systems. The significance of these studies is discussed here with regard to risk analysis and links between extractables and potential leachables in final formulation containers.
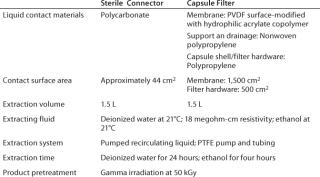
Test Systems and MethodsFor these studies, we specifically selected two single-use components with widely different features to demonstrate differences in the quantity and identity of extractable substances. It also makes clear the wide range of available analytical techniques that enable identification of individual compounds today.
Sterile Connector: Two-piece connectors are used with disposable systems for making sterile connections between two sterile pathways. Once a connection is complete, only one material (polycarbonate) comes into contact with the process fluid, and it has a very low product-contact area (∼44 cm2).
Capsule Filter: The filter capsule we tested incorporates a 0.2-µm sterilizing-grade membrane filter with two major materials that come into contact with process fluid: polypropylene for the filter element and capsule shell and polyvinylidene fluoride (PVDF) for the membrane, which is surface-modified using a hydrophilic acrylate copolymer. The filter has a very high membrane area of 1,500 cm2.
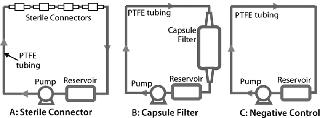
Figure 2 shows test systems for extractables. Table 1 lists product and test parameters. Duplicate tests were performed on each product. The model solvent for dilute aqueous solutions was water, and ethanol represented general organic fluids. Test conditions represented a worst case for most biopharmaceutical applications. Pretreatment with gamma irradiation (50 kGy) also represented an extreme limit of radiation dose used for product sterilization of disposable products and systems. For both fluids and product types, a negative control study was performed using a recirculating system with a polytetrafluoroethylene (PTFE) pump and tubing but with no product in the line.
Table 1: Product and test parameters for extractables studies
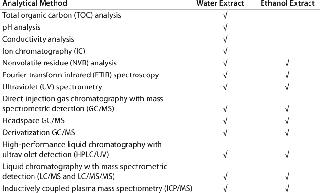
Analytical Methods: We used 13 different analytical methods to assess the quantity and nature of the various chemical species typically associated with these plastic components. As Table 2 shows, most methods were applicable to both water and ethanol extracts, but four were specific to aqueous extractables.
Table 2: Analytical methods used for assessing extractables
Sterile Connector Studies: Because of the small size and low fluid-contact area of the sterile connectors, we incorporated four in series into the recirculation loop to ensure detection of extractable components within the sensitivity limits of the analytical methodologies we used. The study was performed in duplicate. Because of space restrictions, we can’t report here the detailed results in full (information available from Pall Life Sciences on request). However, Table 3 summarizes the most significant results in a concise, simplified form to show qualitative and quantitative data for extractable substances under exaggerated test conditions. We obtained pH and conductivity values from extracts of four connectors. Other values from each individual connector were obtained by dividing by 4 the results from a set of four connectors.
Table 3: Results of extractables studies on sterile connectors (NA = not applicable; DL = detection limit)
The data in Table 3 show that combined nonvolatile residues (NVRs) from four sterile connectors were below the limit of quantification, giving results similar to the negative controls. The contribution to TOC, pH change, and conductivity was minimal. With ion chromatography, formate and acetate from connector extracts were below detectable limits. Water and ethanol extracts analyzed further by high-sensitivity gas chromatography (for volatile and semivolatile extractables) and high-performance liquid chromatography (HPLC) methods showed no differences between the test and control extracts, even after 10- to 50-fold concentration of some samples.
And analysis for 19 elements by inductively coupled plasma mass spectrometry (ICPMS ICP/MS) showed that 15 metals were below detection limits. Only boron, calcium, sodium, and zinc were higher than detection limits (but less than 1.1 ppb) from one connector-based extract.
Bisphenol-A, the primary building block for polycarbonate, has been reported as a leachable substance from some grades of polycarbonate bottles after exposure to heat — e.g., boiling water (5). To assess the possibility of this material leaching from aseptic connectors, we performed a separate ethanol extraction study on four aseptic connectors that were heat treated by autoclaving at 135 °C twice for 30 minutes. No bisphenol-A could be detected by GC/MS in combined extracts from two sets of four connectors.
So the qualitative and quantitative data strongly indicate that the potential for these connectors to release leachable materials into drug products is very low, considering the less vigorous flow conditions and reduced contact times in most processes compared with these extractable test conditions. This conclusion can be important to performing process risk analysis. For cases in which water or ethanol represents a worst-case solvent, the extractables data may indicate that process-specific leachable studies on these connectors could be minimized — or even unnecessary — if full extractables data and risk analysis documentation is available. This can reduce validation effort and time.
Capsule Filter Studies: A single capsule filter was tested in the extraction rig, and this study was performed in duplicate. As stated above, it is not possible here to report detailed results in full (information available from Pall Life Sciences on request). However, Tables 4 and 5 summarize the most significant results in a concise, simplified form to show the general qualitative and quantitative extractables profile data.
Table 4: Nonvolatile residues (NVR) from capsule filters
Table 5: Identity and quantities of some compounds in ethanol filter extracts
The NVRs from aqueous extracts were very low (mean value 5.5 mg). As expected, the ethanol extracts were higher (mean value 56 mg) and in the range typically reported for high-area filters of this type.
Fourier-transform infrared (FTIR) analysis of the NVRs identified them as predominantly oligomers of the acrylate copolymer used for surface-modification of the PVDF membrane. Chromatographic analysis of ethanol extracts to identify individual compounds including both volatile and semivolatile compounds (Table 5) showed traces of fatty acids and antioxidants (additives of the polypropylene resin formulation) and breakdown products of the acrylate copolymer (a membrane hydrophilizing agent) in the range of 0.1 ppm (up to no more than 3 ppm).
DiscussionCapsule filter data showed a much higher level of extractables than was found with the sterile connector. This difference is to be expected because of the greater mass, wider range of materials, and very high surface area of the filters. For example, the pleated hydrophilic PVDF membrane and polypropylene nonwoven support and drainage layers in these capsule filters have a face area of 1,500 cm2 (0.15 m2), about 34× higher than that of the sterile connectors. However, the internal microporous structure of the filter membranes increases their contact area by more than 1,000 times for a total area of >150 m2. In addition, the polypropylene filter capsule shell and hardware have a contact area of ∼500 cm2, which is >10 times higher than that of the sterile connector.
The identities of the compounds we detected are consistent with the basic materials of construction for these components, materials that have been assessed for biological safety during core validation using industry-standard tests such as the US Pharmacopeia’s biological safety class VI for plastics. The materials of construction are also included in each product’s drug master file, which is submitted to the FDA. Furthermore, none of the extractables identified is listed in the harmonized guidance document for residual solvent impurities (7).

Summary and General Conclusions: Our study highlights the value of powerful analytical techniques available today for identifying trace extractables down to parts-per-billion levels. When it comes to leachables in final drug products rather than pure model solvents, the analytical task is more daunting due to interference from active drug ingredients, surfactants, and other nonvolatile components. However, complete worst-case extractables test results from process equipment will facilitate evaluation of leachables in final drug products derived from that process equipment.
Model solvent extractables data (such as those generated in this study) can play an important role in process qualification and validation of single-use systems. The extractables profile of high-area filters will clearly have much more significance in risk assessment for leachables than the much lower results from simpler components such as the sterile connector. With data available from all the components of a system, a scientifically based decision can be made on further qualification programs for the related process. The decision tree in Figure 3 provides a guide to a risk-assessment approach.
If the core data clearly represent a scientifically justified worst case for a process, then extractables data can be incorporated into process qualification/validation documentation. If the solvent and/or test conditions are not justifiable, then a worst-case study on the entire system (scaled down if necessary) can be performed using an appropriate model solvent specific to the process fluid without the need to test components individually.
Further studies are now in progress for intensive analysis of extractables from other individual components as well as complete systems containing flexible biocontainers, tubing, filters, and connectors. These results will be published in a future issue. The combined data will form an important library of information available to end users for selection and qualification/validation of single-use systems.