In a highly anticipated presentation at the 2020 Phacilitate Leaders World event ‚ÄĒ part of Advanced Therapies Week, along with the World Stem Cell Summit in Miami, FL ‚ÄĒ Susan Nichols (chief executive officer for Falcon Therapeutics), highlighted 10 events from 2019 that drove conversation, investment, and innovation in regenerative medicine. Although clustered regularly interspaced short palindromic repeats (CRISPR), business consolidations, and production capacity powered the cell and gene therapy (CGT) space in 2019, a new proactive focus on patient…
Search Results for: zOLGENSMA
Staffing: Cell and gene therapy sector needs reinforcements
Cell and gene therapy manufacturers need staff with laboratory skills and GMP know-how, according to an expert from Texas A&M. Cell and gene therapies attract a lot of attention. In recent years products like Yescarta, Kymriah and Zolgensma and the debate about their prices ‚Äď $373,000, $475,000 and $2.1m respectively ‚Äď have dominated the headlines. In industry circles the focus has shifted to the manufacture of such therapies. Developers are working to ensure there are sufficient vectors, or to make…
AveXis ‚Äėflexible‚Äô NC plant a step closer to fully internal gene therapy network
AveXis has opened a gene therapy plant in Durham it says will be able to produce up to seven products simultaneously. President Dave Lennon talks timelines, capacity, and taking production inhouse. AveXis and its owner Novartis have invested a total of $115 million (‚ā¨106 million) into a site in Durham, North Carolina to support the production of US Food and Drug Administration (FDA) approved product Zolgensma (onasemnogene abeparvovec) and its pipeline of gene therapy candidates. With the doors opening last…
CAR-T at the Crossroads: Is Allogeneic the Way to Go?
As cell therapies move through the clinic toward commercialization, respondents to an Informa Connect industry survey are beginning to look to allogeneic ‚ÄĒ or off-the-shelf ‚ÄĒ products as ‚Äúthe next big thing.‚ÄĚ Almost 200 people contributed to the Cell Therapy Analytics Report, revealing their current positions within the burgeoning cell and gene therapy space and offering their thoughts and predictions for the future. Most survey respondents work within companies developing oncology products. Of those, the largest group (41%) said that…
Repligen fighting fit in $10bn bioprocess sector, eyes CGT for growth
Repligen says planned tech launches and growing cell and gene therapy sector demand will drive market expansion and growth in 2020. The bioprocess and life science tech supplier shared its thoughts at the JP Morgan Healthcare conference. CEO Tony Hunt said a ‚Äúsurge‚ÄĚ in clinical activity is driving expansion in the CDMO space ‚Äď citing announcements by Catalent and Thermo Fisher Scientific ‚Äď and at large pharma firms like Amgen, Novartis and BeiGene. ‚ÄúIf you go back a few years…
Top 10 advanced therapy milestones of 2019: Patient access takes center stage
CRISPR, capacity, and consolidation powered the cell and gene therapy space in 2019, but a proactive focus on patient access topped Falcon Therapeutics CEO Susan Nichols‚Äô annual roundup. In what has become one of the most anticipated presentations at the Phacilitate conference, Susan Nichols, CEO of cell therapy firm Falcon Therapeutics, laid out the top 10 events of the previous year that have shaped the regenerative medicine space, driving conversation, investment, and innovation. The top spot in 2019 focused on…
Phacilitate 2020: FDA commercial cell and gene therapy forecast ‚Äėunlikely‚Äô
Manufacturing issues and a scarcity of new commercial products leave predictions that 10-20 cell and gene therapy approvals each year by 2025 somewhat fanciful, says Dark Horse Consulting. In his plenary address at the Phacilitate conference yesterday, Anthony Davies, founder of cell and gene therapy specialist firm Dark Horse Consulting, reflected on the difficulties the sector has faced since the high of 2017 when three products achieved US Food and Drug Administration (FDA) approval: Kymriah (tisagenlecleucel) and Yescarta (axicabtagene ciloleucel),…
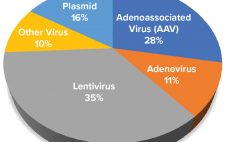
Capacity Analysis for Viral Vector Manufacturing: Is There Enough?
Advanced therapy medicinal products (ATMPs) are engineered to replace defective, disease-causing genes to compensate directly for a genetic defect or to encode a therapeutic protein construct (e.g., chimeric antigen receptor, CAR) for disease treatment. In most instances, a viral vector delivers the engineered genetic payload, targeting cells in situ or ex vivo through cellular modification, expansion, and infusion into a patient. Clinical successes of ATMPs bolstered by regulatory approval of products such as Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), Kymriah (tisagenlecleucel,…
Measure Twice, Treat Once: Navigating the Regulatory Landscape of Assay Development to Ensure High-Quality CGT Products
Cell and gene therapies (CGTs) are a novel and fast-growing class of transformative therapies designed to address gaps in traditional treatment strategies of some of the most severe diseases. By definition, gene therapy ‚Äúseeks to modify or manipulate expression of a gene to alter the biological properties of living cells for therapeutic use‚ÄĚ (1). That can be either an in vivo delivery of a gene or delivery of a gene to a patient‚Äôs cells that are manipulated outside of the…
AAV Vector Manufacturing Platform Selection and Product Development
Adenoassociated virus (AAV) vectors have emerged as the prominent delivery mechanisms of corrective gene therapies. Three such products ‚ÄĒ Glybera (alipogene tiparvovec, uniQure), Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), and Zolgensma (onasemnogene abeparvovec-xioi, AveXis) ‚ÄĒ have been licensed, and a growing number of candidates are entering late-stage development. In mapping out an AAV gene therapy product development strategy, biomanufacturers should address fundamental considerations for their manufacturing strategies for both phase 1‚Äď2 clinical evaluation and translation for commercial market supply. A manufacturing…