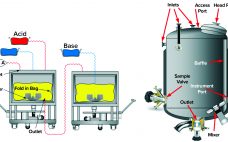
Filtration of protein-based biologics is essential for minimizing viral contamination and ensuring product safety and high quality. The tendency of therapeutic monoclonal antibodies (MAbs) and recombinant proteins to aggregate under a number of conditions can complicate selection of a virus filter. An increasing demand for high concentration formulations creates additional challenges. When performing filterability studies and to ensure meaningful virus filter evaluations, downstream process scientists must address factors that can lead to aggregation. This special report on virus filtration by…
Viral Clearance
Viral Safety of Viral Vectors:
Special Concerns Arise When the Virus Is the Product
As anyone who has focused on host-cell proteins as process contaminants can tell you, trying to purify a specific type of molecule from a large mixture of many similar molecules is like trying to find a few particular needles in a huge pile of varied needles. The same could be said for purifying viral vectors from cell culture fluids. When viruses are the products, unwanted viruses are contaminants that must be separated away — or better yet, prevented from being…
MAb Viral Clearance Studies:
A Substantiated Platform Approach for the IND Stage
Virus removal/inactivation is a major concern in the safety of monoclonal antibodies (MAbs) and other recombinant-protein drugs. Some methods (such as nanofiltration and low-pH inactivation) have been demonstrated repeatedly by the industry to be reliable for most viruses, with >4 log10 removal. Based on my company’s virus-removal experiences with its MAb downstream-process platform, we propose a “bracketing method” — testing only samples that lie at the extremes of a design space — to prove proactively that small differences in operating…
Predicting Viral Clearance in Downstream Process Development
As viruses can arise during the manufacture of biopharmaceuticals, regulatory agencies require viral clearance validation studies for each biopharmaceutical prior to approval. These studies are typically conducted in biosafety level (BSL)-2 facilities and require large capital and human resources. The use of an accurate, economical, and quantifiable noninfectious viral surrogate would enable downstream purification scientists to study viral clearance throughout process development. This report explores the use of a BSL-1 compatible, noninfectious MVM particles to predict viral clearance results over…
Virus Assay Variation Is the Main Source of Variation in Viral Clearance Studies: Retrospective Analysis of a Large Data Set
Biopharmaceuticals produced from mammalian cell cultures are susceptible to viral contamination. That risk is mitigated by applying complementary approaches. Those include extensive testing of cell banks, selecting low-risk raw materials, testing cultivations for viruses, and documenting the capacity of a purification process to inactivate and remove viral contaminants. The latter commonly is referred to as viral clearance and usually expressed as a log reduction value (LRV). Novo Nordisk has performed several viral clearance studies for different processes and process steps.…
Viral Clearance in a Downstream AAV Process: Case Study Using a Model Virus Panel and a Noninfectious Surrogate
Over the past decade, adenoassociated virus (AAV) vectors have become established as leading gene-delivery vehicles. In 2017, the pipeline for gene therapies included 351 drugs in clinical trials and 316 in preclinical development (1–4). As those candidates advance, significant efforts are being made in process development and manufacturing for viral vectors, with the overall goal of reducing process impurities while maintaining the highest possible process yield. To address that goal, industry suppliers have developed innovative AAV-specific separation technologies. Thermo Fisher…
Customized Yeast HCP Quantification with Biolayer Interferometry Using a Horseradish Peroxidase Substrate
Biopharmaceuticals are the largest group of drugs under development (1), and the demand for new and safe drug products is high. The most common bacterial and mammalian cell lines for production are Escherichia coli, Chinese hamster ovary (CHO) cells, and yeast. During a production bioprocess, a cell line expresses not only the molecule of interest, but also host-cell proteins (HCPs). They are considered to be impurities in a final drug product because they can affect the efficacy and safety of…
Ask the Expert: Predicting Viral Clearance During Downstream Development
Until recently, downstream process development (PD) teams have lacked methods for easy, effective, and economical estimation of a process’s viral clearance capability. David Cetlin (senior director of R&D at Cygnus Technologies) delivered an “Ask the Expert” presentation on 13 October 2020 describing how MockV kits containing noninfectious mock-virus particles (MVPs) could fill that gap. Cetlin’s Presentation Viral clearance studies tend to be outsourced to contract research organizations (CROs) because they require biosafety level (BSL)-2 and -3 facilities for working with…
How Much Harm Can a Single Droplet Do? Considerations for a Viral Inactivation Step
Viral clearance is a fundamental aspect of viral safety for biopharmaceutical products. Regulatory agencies around the world require biomanufacturers to segregate their operations appropriately to mitigate the risks of carryover contamination from previous process steps or product batches and of crossover contamination between product(s) made in the same facility. Guidelines are vague in defining “appropriate,” leaving biomanufacturers to interpret regulatory expectations and define their own virus reduction and segregation strategies. Given the differences among manufacturing processes and facilities housing such…
Streamlined Polishing and Viral Clearance Using a New Hybrid, Biomimetic, Single-Use Anion Exchanger
Flow-through anion-exchange (AEX) chromatography is used frequently in biopharmaceutical purification processes for reduction of net–negatively charged host-cell proteins (HCPs) and viruses as part of a validated viral clearance strategy (1, 2). AEX column chromatography is the technology most often used for electrostatic viral clearance, particularly in commercial-scale biopharmaceutical manufacturing, for which columns have a long-established history of reliable and well-understood performance (3). Still, validation of HCP and viral clearance by AEX columns in biopharmaceutical processes involves complexities that contribute significantly…