Sanofi says it is looking to resolve the âpatchyâ accomplishments in the gene therapy space through a new specialized unit that will leverage technologies from its vaccine division. Sanofi has dabbled in the cell and gene therapy over the past decade but is yet to make its mark. Speaking at the JP Morgan Healthcare Conference this week, CEO Paul Hudson highlighted the importance of the sector going forward but admitted industryâs success in the sector has âbeen patchy.â He told…
Search Results for: zOLGENSMA
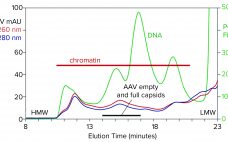
Streamlining Industrial Purification of Adeno-Associated Virus
With its first licensed therapeutic now marketed worldwide (1), adeno-associated virus (AAV) has become a preferred vector for gene therapy. However, unlocking its full potential still poses challenges, many of which are associated with purification. The first involves the transition from upstream to downstream processes. AAV-bearing lysates are laden with debris that foul filtration media and limit or prevent concentration. Another challenge involves reduction of soluble host-cell DNA, which is complicated by its strong association with nucleoproteins. A third involves…
Eli Lilly $880m Prevail buy a âgood entry pointâ into gene therapy
As Eli Lilly agrees to buy Prevail Therapeutics adding a pipeline of neuroscience assets, CEO Dave Ricks hints at further acquisitions in the gene therapy space. The deal announced yesterday will see Eli Lilly pay approximately $880 million upfront for New York-based Prevail Therapeutics, with a further $160 million set to be paid upon the first approval of a product. Prevail, launched in 2017, is developing gene therapies to slow or stop the underlying disease process for patients with neurodegenerative…
Catalent reiterates confidence in the gene therapy sector
Gene therapies will help drive growth says Catalent, which reiterated its confidence despite recent setbacks in the sector. The contract development and manufacturing organization (CDMO) spoke about its growing gene therapy business during its first quarter earning call last week, explaining it is seeing ongoing âelevated demandâ for this type of work. Revenue generated by Catalentâs biologics business â which covers biopharmaceutical manufacturing as well as cell and gene therapy production â was $377.1 million in the first quarter of…
Roche enlists Dyno to bring AI to liver, CNS gene therapies
Roche hopes to bolster its capabilities in liver and central nervous system (CNS) disorder gene therapies by turning to Dyno Therapeutics. Roche made a splash with its acquisition of Spark Therapeutics, whose Luxturna (voretigene neparvovec), a gene therapy for an inherited form of vision loss, was the first such therapy to win FDA approval. Dyno, a two-year-old startup based in Cambridge, Massachusetts meanwhile, has never brought a gene therapy to the market, nor does it currently have its own drug…
Sartorius set to acquire BIA Separations for $423m
Sartorius Stedim Biotech has announced plans to buy Slovenian purification tech firm BIA Separations for â¬360 million ($423 million). The deal will see Sartorius pay â¬240 million upfront and â¬120 million in shares. The firm will also pay tranches of earn-out payments over the next five years. The transaction is expected to complete before the end of the year. AjdovÅ¡Äina, Slovenia-headquartered BIA develops and manufactures products for purification and analysis of large biomolecules, including viruses, plasmids and mRNA. Sartorius CEO…
Adios AveXis: Novartis embeds $8.7bn acquisition into gene therapy rebrand
Gene therapy developer AveXis will be known as Novartis Gene Therapies, more than two years after being acquired by the Swiss biopharma giant. In May 2018, Novartis spent $8.7 billion to acquire gene therapy technology and therapy developer AveXis. Since then, Novartis has supported the commercialization of its spinal muscular atrophy (SMA) single-dose therapy Zolgensma (onasemnogene abeparvovec), while investing heavily into the bolt-ons manufacturing capabilities. In February, AveXis/Novartis opened a $115 million gene therapy production plant in Durham, North Carolina.…
Viral-Vectored Gene Therapies: Harnessing Their Potential Through Scalable, Reproducible Manufacturing Processes
We might not associate the jazz queen Ella Fitzgerald with 21st-century gene-based therapies, but the First Lady of Song was on to something back in 1939 when she sang ââTâAinât What You Do (Itâs the Way That You Do It).â Although demonstrating the safety and efficacy of gene-based therapies in rigorous clinical trials is essential for gaining product approval from regulators, doing the bare minimum is insufficient. The way that such products are produced also matters. Manufacturing processes and protocols…
AveXis redeploying CDMO gene therapy capacity to support COVID-19 project
The Novartis gene therapy business will contribute its technology and dedicated space at a facility run by CDMO Catalent to help produce a COVID-19 vaccine being developed by Massachusetts-based researchers. Massachusetts Eye and Ear and Massachusetts General Hospital have teamed to develop a vaccine candidate that uses an adeno-associated virus (AAV) vector to deliver and express the Spike gene of the SARS-CoV-2 virus, which causes COVID-19, to elicit an immune response. The developers have turned to AveXis, the gene therapy division…
Cell and Gene Therapies Get a Reality Check: A Conversation with Anthony Davies of Dark Horse Consulting Group
As founder of cell and gene therapy (CGT) specialist firm Dark Horse Consulting Group in California, Anthony Davies speaks from a quarter century of experience including former positions at Onyx Pharmaceuticals, Syrrx, ZymeQuest, Serologicals, Geron Corporation, Capricor, and 4D Molecular Therapeutics â and he currently serves on the board of directors for TrakCel and the scientific advisory boards for Akron Biotech and BioLife Solutions. In his plenary address at the Phacilitate 2020 Leaders World conference (part of Advanced Therapies Week…