Cell substrates managed in controlled culture environments have become, over the past few decades, the subject of intensive technological developments for the biomanufacturing of viral vaccines. The driving force of such work is an expanding demand for safety, high production capacities, cost savings, and flexibility. Egg, tissue, and primary-cell–based manufacturing methods of limited capacity are now considered to be outdated technologies.
In the influenza vaccine field, for example, time delays in vaccine delivery (especially during pandemic responses) have increased concerns over whether the classic egg-based production system is capable of meeting increasing demands. Hence, manufacturing with cell culture platforms offers high flexibility and optimal scaling capacities, important features for guaranteeing vaccine supply.
Demonstrating the expanding interest for such technologies, several cell-based influenza vaccines have been recently approved for market (1). Beyond the influenza vaccine example, nearly 400 vaccines are reported to be in development (2). Many of those pipeline products involve cell-based technologies in producing improved second-generation vaccines and new products for diseases for which no associated vaccines are currently available.
Addressing Challenges of Vaccine Production
Like virus production with adherent cells, many new vaccines in development are using new cell substrates. To adapt to today’s market needs, cell-based vaccine production processes are inherently associated with a combination of technological advancements that include novel expression systems, improved cell culture media, modular/transportable bioprocessing facilities, and single-use technologies, especially single-use bioreactors. A number of single-use bioreactor designs are commercially available. It is important to assess and qualify those multiple designs to ascertain what will effectively address the challenges associated with production of cell-based vaccines:
- Predictable scalability with adherent and suspension cells,
- Design that reduces the number of bioreactors in the seed train (3),
- Design that enhances biosafety and reduces contamination risks,
- Ergonomics of single-use bags, system automation, and software for cell culture.
As a technological solution to support modern vaccine development, single-use bioreactors offer the operational flexibility for suspension-or adherent-cell processes from bench (2 L) to industrial production scale (≤2,000 L). Biosafety is enhanced by specific design features such as leak testing of each assembly, a device for reliable sensor insertion, and an oversized exhaust filter to prevent overpressure in bags. However, selection of a single-use bioreactor cannot be disconnected from the cell expression system used to produce a vaccine.
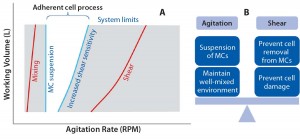
Cell Culture on Microcarriers and Challenges for Process Development: Most cell culture processes for vaccine applications are mainly based on adherent cells: e.g., MRC5 human lung cells, Vero simian kidney epithelial cells, Madin-Darby canine kidney (MDCK) cells, and chicken embryo fibroblast (CEF) cells. In a stirred bioreactor environment, such cells can be grown on microcarrier supports in suspension, which provide solid surfaces on which cells can attach and proliferate. One of the biggest challenges with such technology is to prevent the sedimentation of microcarrier beads or discs without dramatically exposing cells to shear forces if high agitation speeds need to be applied. Optimal stirring conditions required for microcarrier systems must be determined experimentally and addressed case by case because many bioreactors have unique impeller designs (4). An operating window needs to be determined within which agitation rates allow for sufficient microcarrier suspension without damaging cells. Bulk liquid mixing must balance the level of hydrodynamic shear (Figure 1).
In particular, studies have demonstrated that adherent cells grown on microcarriers are highly sensitive to hydrodynamic shear forces, which can significantly reduce the upper end of the operating window (5–7). Optimized adherent-cell cultures have been established in single-use bioreactors as Figure 2 shows for Vero cells. However, handling such cell substrates at the industrial level requires underlying cumbersome, time-consuming, and costly process-development efforts to determine a design space for optimal use.

Figure 2: Optimized vaccine manufacturing process using adherent cells in single-use bioreactors (Vero, 200-L scale) (3)
Success of Suspension Cell Lines at the Regulatory Level: Because of the complexity and challenges linked to culturing adherent cells in a bioreactor, alternative expression systems are emerging that use suspension cell cultures, as exemplified by recent successes at the regulatory level. In 2007, Optaflu seasonal influenza vaccine from Novartis (produced in MDCK cells) was approved for the US market. Later, the EB66 duck embryonic stem-cell line received market authorization from Japanese health authorities for H5N1 influenza vaccine production (8).
Production of Measles Virus
Derived from duck embryonic stem cells, the EB66 suspension cell line from Valneva is highly permissive to a broad range of human and veterinary viruses, and it displays regulatory and industrial-compliant characteristics (9). Today, more than 30 vaccine manufacturing programs involving about 20 veterinary and human health biopharmaceutical companies are dynamically progressing in upstream process development using this cell substrate. These cells propagate in all tested manufacturing systems, with successful up-scaling up to 1,000 L using conventional stainless-steel bioreactor systems.
For several years, growing interest has been given to single-use bioreactor systems as an option for developing robust upstream vaccine production processes with high simplicity, flexibility, and cost-effectiveness. A first generation of cell culture production process was developed using the EB66 platform for production of a recombinant, live, attenuated Schwartz-Measles virus (kindly provided by Frédéric Tangy, head of the viral genomic and vaccination laboratory at Pasteur Institute in Paris, France). That process was used as a model for evaluation of EMD Millipore’s Mobius disposable bioreactors. Here we report success in transferring and up-scaling EB66 cell growth and viral vaccine production from conventional bench-top glass bioreactors to disposable 3-L and 50-L single-use bioreactor systems (Figure 3).
Our major objective was to transfer and scale up a first-generation upstream process into a single-use bioreactor environment by applying two distinct types of vessel. Materials installation was quick and simple enough to allow for inoculation of the bioreactor bags within one week after their receipt. No bioreactor autoclaving was necessary with the 3-L system, and it was compatible with a third-party controller system (from Applikon). To perform the culture, we preserved all parameters formerly established in glass systems, including the proportional/integral/ derivative (PID) settings. The 50-L system’s rigid base allowed for straightforward one-way bag installation, which significantly simplified the preparation step compared with other single-use systems.
With respect to the bioreactor set-up, specific parameters — e.g., geometry, impeller design, tip speed, power input per volume (P/V), and oxygen mass transfer coefficient (kLa) — have proven useful in allowing for an optimal scale-up from benchtop to pilot-scale bioreactors. For our first approach, we decided to focus on agitation alone. Applying a P/V value of 2.4 W/m3 calculated from the 3-L single-use run to the 50-L scale resulted in agitation speed modification. For PID settings, we used established values.
The upstream viral-production process consists first of biomass accumulation over a three-day period in a volume of culture medium representing one third of the process volume capacity. Next, we inoculate EB66 cells at 0.4 × 106 viable cells/mL and grow them at 37 °C and pH 7.2, with 50% dissolved oxygen. For the second phase of the process, cells are directly infected at an appropriate multiplicity of infection (MoI). At the same time, we shift the temperature to 33 °C and fill the bioreactor with 2/3 volumes of a second culture medium, which was selected to support viral spread through the EB66 cell population.
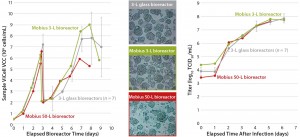
Figure 4: EB66 cell growth and morphology (left, center) and measles virus productivities (right) in Mobius 3-L and 50-L single-use bioreactors
During the course of the experiment, we monitored cell growth and morphology as well as metabolite profiles. At the end, we analyzed viral production yields and compared those with results previously obtained using benchtop 3-L glass bioreactors. We saw consistent cell growth in both single-use bioreactors compared with reference data (Figure 4, left). Expected metabolite profiles and regulation of physical parameters were optimal for cultures performed in both single-use devices (data not shown). Consistently, light microscopic observation of the cells revealed no undesired behavior for EB66 cells placed in this new culture environment (Figure 4, center). Finally, infectious viral titers reached the targeted yield (Figure 4, right), demonstrating scalability of the Mobius bioreactors for EB66 cell growth and optimal measles virus production. An interesting aspect of this study was the sufficiency of the P/V parameter for an effective process up-scaling from 3 L to 50 L.
Economies of Scale and Cost Savings
Key characteristics of EB66 cells are their strong resistance to high dilution ratios and short population doubling time in culture (~16 hours). Small quantities of these cells are needed to dilute at the seeding step, so they can reach high cell densities in a short period. EB66 cells also offer favorable cell culture conditions, making high industrial scales feasible, as already demonstrated in 1,000-L stainless steel bioreactors (data not shown). To further elaborate on and evaluate the advantages of combining EB66 cells with single-use bioreactors at the industrial level, we performed an up-scaling calculation analysis, including the complete set of Mobius systems. Figure 5 shows two process scenarios using 200-L and 2,000-L bioreactors.
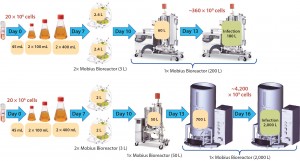
Figure 5: High-scale virus production process overview with Mobius single-use bioreactor systems; (top) suspension EB66 cells at 200-L scale; (bottom) same process at 2,000-L scale
As Figure 5 shows, a reduced cell culture time step characterizes both processes. Times to cell infection were 13 and 16 days at the 200-L and 2,000- L scale, respectively. For these processes, no intermediate media exchange steps or labor-intensive cell detachment/attachment operations are required, as are needed for adherent-cell systems using microcarriers. In addition, EB66 cells’ resistance to high dilutions (combined with the 5:1 volume turndown ratio of the Mobius bioreactors) allows for a seed train bioreactor quantity and size reduction (one 50-L bioreactor can seed a 2,000-L bioreactor). So we achieved an overall reduction of cost and process complexity for large-scale manufacturing using these suspension cells.

Figure 6: Cost of each GMP cell culture campaign (from cell thawing to virus infection) with EMD Millipore Mobius single-use assemblies and EB66 cells (calculated with BioSolve software)
Next, we performed a direct cost comparison between EB66 and Vero cells, from thawing step to infection (Figure 6). Using BioSolve software from Biopharm Services, we ran two analyses considering cost break-downs by materials, consumables, production labor, and facility use. (Labor and facility costs were calculated with default values, but we did not take capital charges or QA/QC costs into account.) Analysis 1 (Figure 6a) compared EB66 and Vero cell culture processes for virus production in a 200-L single-use bioreactor, evaluating the impact of each cell substrate on total cost. Analysis 2 (Figure 6b) compared EB66 cell culture processes for virus production in 200-L and 2,000-L single-use bioreactors, evaluating the economies of scale.
The outcome of Analysis 1 shows a cost reduction of ≤40% with the EB66 cell line compared with a typical adherent-cell process in single-use bioreactors (Figure 6a). Most of those savings come from dominating cost reductions made possible by process simplification: –30% production labor cost and –69% raw materials cost. Figure 6b shows the costs of a 2,000-L cell culture campaign to be 2.5× higher than the 200-L campaign, mainly due to an increase in materials cost (particularly cell culture media and supplements). At the 2,000-L scale, materials become the largest cost contributor (50% of total cost), whereas consumables, production labor, and facility have a lower impact on total cost than at the smaller scale.
Combining EB66 cells with an EMD Millipore Mobius single-use bioreactor platform from 3 L to 2,000 L offers an attractive solution for simple and cost-effective vaccine production. This platform gives manufacturers the ability to produce high-yield and -quality vaccines in a reduced timeframe compared with previous technologies, hence matching the market need for a cost-saving and safe vaccine production method.
References
1 Influenza Vaccines for the Future, Second Edition. Rappuoli R, Del Giudice G, Eds. Springer: Basel, Switzerland, 2011; 300–301.
2 Medicines in Development: Vaccines — A Report on the Prevention and Treatment of Disease Through Vaccines. Pharmaceutical Research and Manufacturers of America: Washington, DC, April 2013; www.phrma.org/sites/default/files/pdf/Vaccines_2013.pdf.
3 Calvosa E, Seve N. US 20110151506 A1: Process for Culture of Adherent Cells. Sanofi Pasteur SA, 23 June 2011; www.google.com/patents/US20110151506.
4 Der K. Determining Agitation Requirements for Microcarrier Processes: Method Development in the Mobius CellReady 50L Bioreactor (poster). 2014 BioProcess International Conference and Exhibition: October 2014, Boston, MA. IBC Life Sciences: Westborough, MA.
5 Croughan MS, et al. Hydrodynamic Effects on Animal Cells Grown in Microcarrier Cultures. Biotechnol. Bioeng. 29, 1987: 130–141.
6 Cherry RS, Papoutsakis ET. Physical Mechanisms of Cell Damage in Microcarrier Cell Culture Bioreactors. Biotechnol. Bioeng. 32, 1988: 1001–1101.
7 O’Connor KC, et al. Agitation Effects on Microcarrier and Suspension CHO Cells. Biotechnol. Techniq. 6, 1992: 323–328.
8 Valneva Announces the First Ever Marketing Authorization for a Human Vaccine Produced in the EB66® Cell Line. Valneva SE: Lyon, France, 24 March 2014; www.valneva.com/download.php?dir=News_2014&file=2014-03-24_Valneva_Human_Vaccine_PR_EN.pdf.
9 Brown SW, Mehtali M. The Avian EB66® Cell Line: Application to Vaccines and Therapeutic Protein Production. PDA J. Pharmaceut. Sci. Technol. 64(5) 2010: 419–425. •
Brice Madeline (upstream process engineer at Valneva SE) and corresponding author Sylvain Ribaud (upstream field marketing consultant at Merck KGaA, Frankfurter Strasse 250, 64293 Darmstadt, Germany; sylvain.ribaud@emdgroup.com) contributed equally to this manuscript. Corresponding author Arnaud Léon (arnaud.leon@valneva.com) is head of virology group I at Valneva SE. Alex Xenopoulos is a principal research scientist, and Janice Simler is a senior research scientist at EMD Millipore. Klaus Schwamborn is vice president of discovery research and innovation