As the biopharmaceutical industry continues toward streamlined bioprocessing and intensified cell-culture biology, selection criteria of single-use bioreactors (S.U.B.s) and other bioprocessing technologies will become increasingly rigorous, emphasizing the importance of considering every aspect of technologies under evaluation. In part 1, we discussed performance for process control, including the maintenance of critical process parameters (CPPs), and highlighted bioreactor performance (e.g., mass transfer, power per volume, and temperature control) as a critical consideration during the selection of S.U.B.s (1). Part 2 focuses on physical and operational aspects of Thermo Fisher S.U.B.s, including hardware ergonomics, bioprocessing container flexibility, and quality vendor relationships.
S.U.B. Hardware
Hardware (e.g., shell, skid, control tower, and human–machine interface, HMI) is a key element in dictating the usability, ergonomics, flexibility, and performance of a S.U.B. Thermo Fisher Scientific S.U.B.s are designed with end-user ergonomics in mind, with large doors and porting options for loading of single-use bioprocess containers (BPCs) with ease. The company’s hardware also has BPC-locating tabs and BPC hoists to alleviate end-user strain during installation of disposables and to facilitate consistent and uniform BPC loading (Figure 1B).
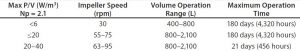
Table 1: Qualified and validated operating ranges for the 2,000-L Thermo Fisher HyPerforma S.U.B. two-piece shaft
A top-mount motor provides power to the unit. The motor interfaces with a robust, qualified, and sterile-validated bearing. A power-transfer shaft is passed through the motor-bearing interface and down through a sleeve to interlock with the impeller. To ensure robustness of power transfer from motor to impeller, Thermo Fisher tested this system extensively across the operating range and conditions of the S.U.B.s, including volumes and power input. Product-life testing has demonstrated robust operation of the power-transfer system, with a safety factor of more than twofold across all vessels when operated according to validated guidelines (Table 1).
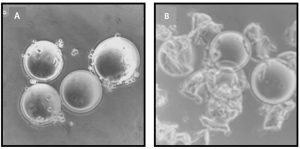
Figure 2: Comparing freshly prepared microcarriers (A) with those run in a system with a bottommount drive (B); Thermo Fisher HyPerforma S.U.B.s prevent this type of damage for adherent-cell culture by keeping mechanically moving parts (e.g., bearings, bushings, impellers, and magnets) off of the bottom of the tank, where settled microcarriers can get caught and destroyed.
The ability to raise the impeller and power-transfer system of the vessel off the floor provides several benefits over alternative systems. The top-load shaft generally enables better mixing performance compared with floor-mounted systems by raising the mixing mechanism off the floor, thus providing unobstructed radial and axial mixing for a more uniform power input top-to-bottom into the system. That uniform power distribution prevents potential column-gradient formation across volumes and scales by minimizing distance between the power input and potential liquid addition ports. The shaft-driven impeller also enables custom and rational designs to be used for a specific operation. For example, custom impeller sizes, locations, and performances have been used for specific applications, including adherent-cell culture, continuous processing and perfusion, and aggressive fed-batch cultures. The top-drive system also is particularly beneficial for highly shear-sensitive systems such as a microcarriers. During microcarrier settling or even during the normal operation of microcarrier-adherent cultures, bottom-mount impellers, bearings, and bushings all can create high-shear zones that lead to poor culture and destruction of microcarriers (Figure 2).
Robust Controller and Peripherals
Thermo S.U.B.s always have been designed under an open-architecture space, allowing integration with any controller option. Although this capability remains, with the recent acquisition of Finesse Solutions, Inc., Thermo Fisher Scientific now can provide a complete bioreactor–controller combination and create workflow solutions that integrate upstream and downstream processes using smart-factory automation.
Those highly configurable controllers offer end users the ability to customize hardware options to a specific bioprocess while offering the ability to transfer configured processes readily across sites and scales for creating a more uniform and consistent bioprocess workflow. Inherent in the configurability of these controllers is the integration of both conventional and single-use sensing technologies and connectivity with third-party devices through supervisory control and data acquisition (SCADA) and open platform communication (OPC). Another consideration during the selection process is an ease and ability to integrate and control peripheral devices, such as temperature control units (TCUs), pumps, and other control-feedback devices.
Disposable BPCs
Key aspects to the performance of any single-use system is the performance, robustness, and ease-of-use of its disposable enclosure or BPC. Most S.U.B. BPCs at pilot and manufacturing scale are made from flexible polymer films. Critical attributes of such films include product contact layer composition, extractables/leachables, robustness against wear and sterilization procedures, and high quality of plastic–plastic welds.
Thermo Fisher’s Aegis 5-14 film has been engineered explicitly with such attributes in mind and qualified for product contact with each layer of the film. As the primary sterile barrier, the film and BPC construction must be robust and reliable to all aspects of operation, from shipping to unpacking, installation, and use. Aegis 5-14 film is designed with multiple layers to maintain the sterile barrier in the face of such wear, including creasing, impacts, and scraping incurred. And the plastic welds used for BPC assembly are designed to be stronger than the film itself and are tested for each lot of BPCs through inspection and burst testing.
Other components that can be integrated into a BPC include port fittings, bearings, and tubing-line sets. All components incorporated into Thermo Fisher S.U.B.s are tested for biocompatibility of both their primary materials and potential extractables. The company continually strives to minimize leachables and extractables in its products and works to identify such materials and understand their possible effects on biological processes.
Although standard BPCs help to reduce costs and streamline operations, flexibility and customization remain desirable for bioreactor systems, particularly with respect to BPCs, as new technologies and needs arise (e.g., new process analytical technologies, PATs, or porting requirements). Thermo Fisher BPCs are highly customizable from a large library of standard qualified parts available for integration, including ports, impellers, and spargers. New parts can be engineered or qualified as a culture technology requires.
Efficient Facility Operations: Warehousing to Disposal
Considerations for S.U.B.s that easily can be overlooked are the facility design and logistics required for them to operate efficiently. Upon arrival at a facility, S.U.B. BPCs will require warehousing. The receiving site should examine the BPCs with regard to packaging size and storage ability. For example, how many BPCs can be stored in a given space and at what cost? Thermo Fisher HyPerforma S.U.B. BPCs up to 2,000 L in scale readily fit on standard US pallets and are under 2 ft in height, allowing for easy warehousing and stacking. That reasonable size combined with appropriate multilayer packaging enables easy movement through and between a facility’s environmentally classified spaces.
A related factor to consider is BPC disposal. For example, is a BPC recyclable or burnable for energy? What fraction is made of metal or other materials that must be separated before disposal, recycling, or incineration? Does the BPC contain materials that are difficult to recycle (e.g., rare earth magnets)? Thermo Fisher S.U.B. BPCs are designed without magnets and with minimal metal to streamline multiple environmentally considerate disposal methods. Furthermore, these BPCs can be compressed readily for predisposal autoclaving or direct insertion into incineration.
Facility layout and design naturally will play a role in efficient facility operation and is an extensive topic that cannot be fully treated here. However, specifically with respect to S.U.B.s, some general aspects should be kept in mind. Physical space often is a premium, both horizontally (square footage) and vertically (ceiling height requirements). That applies to the vessel and extends to its base, control tower, and peripherals, such as HMI and TCU. In terms of flexibility, modular construction (e.g., separate vessel, HMI, and TCU) generally allows for more flexible setups and space use than do units that have the primary vessel and peripheral units integrated into a single item. Unit modularity also can increase unit mobility and simplify setup for product-specific processes. To maximize potential flexibility and meet the range of customer specifications, Thermo Fisher S.U.B.s are separate units from HMI control towers. Furthermore, S.U.B.s up to 1,000 L in scale are available with casters for maximum mobility.
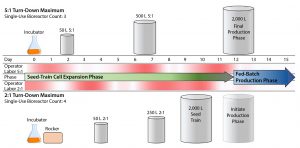
Figure 3: Improving facility efficiency with increased S.U.B. turn-down and careful logistical planning; using high turn-down bioreactors (e.g., 5:1) can enable a streamlined seed-train for cell expansion. Benefits of high turn-down include increased facility space through reduction of bioprocess units, reduction of operator labor (e.g., setups, take-downs, and sterile or aseptic transfers), and reduction of logistical risks. With a bioprocess unit removed (e.g., rocking-motion bioreactor), the cell expansion requires about 15–25% less operator intervention and 20% fewer single-use bioprocess containers. The high-intensity portions of operator labor (dark red) of 5:1 seed train is spaced at intervals of 2–4 days, reducing risk of operator error through fatigue or haste. Using a 5:1 500-L S.U.B. as the n – 1 bioreactor allows a process to spend 3–5 days fewer in the terminal 2,000-L reactor than a 2:1 seed train, promoting efficient use of terminal volume reactors and enabling nimble, flexible campaigns. Maximum split ratio = 5, minimum cell density = 0.3 million cells/mL.
S.U.B. mobility and variable volume operation (or turn-down ratio) facilitate unique opportunities for flexible operations and nimble scheduling. Figure 3 emphasizes some benefits of increased turn-down operations. For example, a 5:1 turn-down (bioreactor can operate at 20% maximum capacity) can reduce the required number of cell-expansion bioreactors, which in turn reduces operator labor and spaces out peak operational labor loads compared with a cell-expansion based on 2:1 turn-down bioreactors. Furthermore, having fewer reactors translates to fewer transfers, fewer setups, and fewer consumables, all of which can help mitigate risks related to sterility and supply assurance. Finally, the possibility of using a 500-L S.U.B. as the n – 1 bioreactor enables the terminal-stage reactor to be in operation for less time per batch, potentially increasing total facility campaigns per year. Thermo Fisher S.U.B.s are capable of 5:1 operation and supporting such flexible bioprocesses.
Quality Operations and Technical Support
As mentioned above, one key aspect of a single-use bioreactor operation is quality, including reliability, robustness, assurance, and logistics of supply. This topic is vast in scope and cannot be fully addressed here. But it should remain a key consideration when selecting a bioreactor or other single-use technology.
From a quality perspective, some highlights to consider include quality management systems, robust design history files, quality experience history, incorporation of quality by design (QbD), implementation of statistical process control, and risk mitigation strategies (e.g., design failure modes and effects analysis, DFMEA). From an operational perspective, some highlights for consideration are supplier relations; assurance of supply; technical and quality supplier support; and product shipping, storage, and movement logistics. As an organization, Thermo Fisher strives to develop good supplier partnerships with strong technical and quality personnel to meet consumer needs. Products are engineered with QbD and supported with a full package of S.U.B. performance characterization and validation data. Furthermore, Thermo Fisher Scientific offers extended characterization and process development services to meet specific consumer needs.
S.U.B.s Evolving for Evolving Needs
Since its initial launch in a market dominated by batch processes, the Thermo Fisher S.U.B has supported biopharmaceutical partners around the world. As processes have progressed to fed-batches achieving fivefold increases in cell density and 20-fold increases in productivity, the company has deployed novel technologies such as the Thermo Scientific drilled hole sparger (DHS) to provide consistent solutions for consumers across the 50–2,000-L scale. The excellent performance of S.U.B. technology has been matched by a devotion to quality, reliability, and robustness in BPC manufacturing to minimize bioprocess risks. As processes continue to evolve toward more aggressive cultures, enhancements to current S.U.B. designs can be made to improve outcomes for process-specific applications (1). For example, increased power input in smaller vessels provides a useful tool for high-density perfusion cultures, and low-speed and lowered impeller customizations can benefit microcarrier cultures.
Further advances in bioprocessing will lead to even greater cell densities and productivities. This will necessitate increased agitation powers to handle raised viscosities and even greater oxygen and carbon dioxide mass-transfer rates. Improved understanding of the effect of nutrient supplies, feeding strategies, temperature shifts, and control parameters on critical product quality attributes will place even more demand on S.U.B.s, sensors, and associated controllers. Thermo Fisher Scientific remains dedicated to innovation and collaboration in developing solutions for the next chapter of bioproduction.
Acknowledgments
The authors acknowledge the many contributors to the data and discussion provided in this work, including Rick Hendrickson, Matthew Summers, and Jordan Cobia for their assistance in data collection and analysis, and the many industry collaborators who provide the essential industrial perspective.
References
1 Smith MT, et al. Single-Use Bioreactors: Performance and Usability Considerations, Part 1 — Performance and Process Control. BioProcess Int. 16(6) 2018: i15–i19.
2 The Technology Roadmap for the Biopharmaceutical Manufacturing Industry — Process Technologies. BioPhorum Operations Group: Sheffield, UK, 2017.
Corresponding author Dr. Mark T. Smith (mark.smith3@thermofisher.com) and Dr. Tony W. Hsiao are senior engineers in the BioProcessing Single-Use Technologies Advanced Technology Research Group at Thermo Fisher Scientific. Dr. Benjamin Madsen is senior engineer in the BioProcessing Single-Use Technologies Process Development Group at Thermo Fisher Scientific. Nephi Jones is the senior manager over applications research in the Bioprocessing Single-Use Technologies R&D at Thermo Fisher Scientific.
For further information regarding the Thermo Fisher HyPerforma S.U.B. system or other Thermo Fisher SUT products, please visit our website at https://www.thermofisher.com/single-use-technologies or contact the authors.
© 2018 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.

