The Bolt-on Bioreactor (BoB) project is an independent initiative aimed at developing and commercializing a bioreactor for efficient, automated culture of adherent cells in production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges. The first challenge has to do with volumetric productivity, the second with process automation, the third with containment and sterility, and the fourth with process economics. This is the first of four articles addressing each of those challenges and describing design features incorporated into the BoB project in development to overcome them.
Challenge #1: Herein I address the first challenge: to develop a system that provides a huge surface area for optimum cell attachment in a reduced volume. The surface should be well suited for cell attachment. The culture area should be enough to produce at least 10 times as many cells as standard devices can. The size of this culture device should be such that it can be easily handled by a technician in a laboratory furnished with standard equipment.
Adherent cells need a surface on which to attach themselves to grow and multiply. So the straightforward strategy for increasing the number of cultured adherent cells is to increase the available surface area on which they can attach. The strategy is similar to that for suspension-cell culture, in which ever- larger culture vessels produce larger batches. But for suspension-cell culture, volume is increased after having optimized volumetric productivity. In other words, before the vessel volume is increased, the culture process is optimized to obtain as much product per liter as possible. Optimized adherent-cell culture processes do exist to maximize productivity per square centimeter. But standard culture devices do not provide for optimal use of available volume because only a fraction of the space taken up by a culture device is a surface made available for cell attachment.
For example, consider roller bottles. Only the inner area of the cylindrical bottle wall is available for cell attachment. The situation is worse with T flasks and culture plates, in which only the inner surface of the bottom of each device is colonized by cells.
When the BoB team considered how to solve this first challenge, some strategies came to mind. The most efficient strategy regarding surface area seems to be to introduce particulated supports within a culture device, thus providing an enormous amount of surface area on those small particles within a vessel. Another strategy is to stack culture plates within a device, thus making a better use of space and lessening repeated processing steps such as culture-medium exchange. Yet another strategy is to roll a large, continuous culture membrane within a device, thus providing a continuous surface that can be fully colonized by dividing cells. Less obvious is the use of hollow fibers bundled together and encased within a cartridge so that cells can grow on their outer surfaces while fresh medium circulates through the lumina of the fibers. For all these strategies to increase attachment surface area (Figure 1), single-use versions are already commercially available.
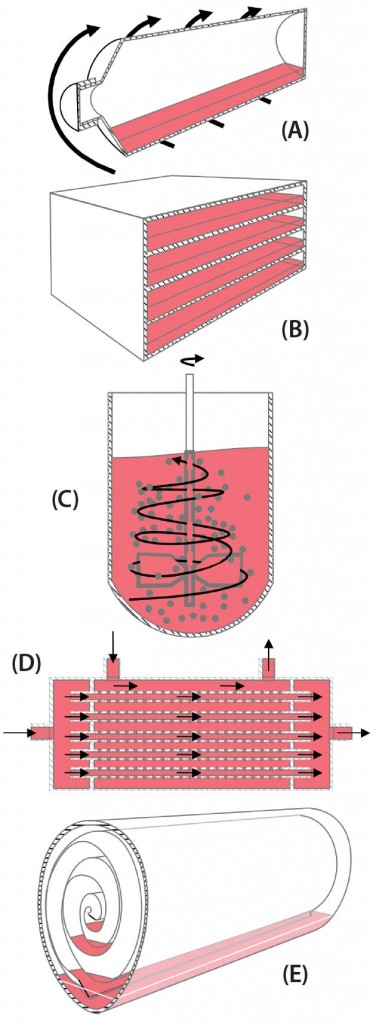
Figure 1: Schematic representations of five available strategies for increasing volumetric productivity in adherent cell culture — (a) roller bottle, (b) plate stack, (c) particulated supports (microcarriers), (d) hollow-fiber bioreactor, and (e) rolled membrane
Particulated Supports
Particulated supports are commonly referred to as microcarriers. They can be used in fluidized-bed mode (e.g., Pall SoloHill and GE Healthcare Cytodex microcarriers) or in packed-bed mode (e.g., Fibra-Cel disks by Eppendorf and iCELLis system by Pall). Using particulated supports for cell attachment provides a huge surface area within a given culture vessel. Automation and continuous cultures can be achieved easily using the same equipment familiar to suspension cell culture for many years. However, using particulated supports for adherent-cell culture does have some drawbacks.
Fluid-Bed Mode: How do cells from loaded particles colonize cell-free particles? In traditional culture devices for adherent cells (e.g., roller bottles), cells simply divide and grow one by another, then end up colonizing the whole wet area available for attachment. So a relatively small cell population can colonize a relatively large culture area. However, when using particulated supports for adherent cell culture, every particle needs to be colonized with some cells at the start of the process because chances are slim for cells moving from one particle to colonize another (2). In practice, this makes the number of cells needed for initial seeding relatively large.
Here are some other considerations:
- Because support particles are mechanically suspended, most of a culture vessel must be filled continuously with culture medium. Apart from considerations regarding medium use, gas transfer to growing cells becomes a bottleneck (3).
- Vigorous agitation provokes foaming and particle collision, exposing cells to shear stress (4).
- Agitation of culture medium with an impeller will cause fluid-speed gradients within a vessel, which in turn produces a nonhomogeneous distribution of support particles (5). The same effect (to a lower extent) can be expected with media shaking in rocking platforms (6).
In packed-bed operated systems, the culture environment is not homogeneous throughout a microcarrier bed. The major cause for such heterogeneous distribution is gas transfer. In these systems, culture medium is efficiently oxygenated before it enters the packed bed, but as medium progresses through a bed, it becomes rapidly depleted of oxygen, creating a strong gradient of dissolved oxygen (7). Harvesting cells from packed beds is also an inefficient step in the culture process (8).
Plate Stacks
There is a difference between producing cells or using them as “factories” to produce valuable substances (e.g., protein therapeutics). Plate stacks operating in batch mode (e.g., Corning CellSTACK and Thermo Scientific Cell Factory systems) has become a preferred option for many users growing allogeneic therapeutic cells (9). The reasons are clear: Traditional culture plates and T flasks provide a reliable surface on which to culture adherent cells, and by using plate stacks you multiply available surface while eliminating some task repetition. A more productive adaptation of this strategy is Pall’s Integrity Xpansion system, in which a multiple-dish system operates in perfusion mode.
Despite the advantages of plate stacks over T flasks and culture plates, the BoB team encountered some concerns when considering them as a solution to Challenge #1. Cells multiplying on one plate do not colonize other plates in a stack because each plate behaves as an independent culture, with a common inlet and outlet with the others. Therefore, every plate must be seeded at the same cell density to obtain similar growth on every one. Media use is not improved over that seen with traditional plates because every plate in a stack requires the same volume of medium that it would have required if it were cultured independently.
Even when as many step repetitions are eliminated as there are plates in a stack, handling stacks can be cumbersome because increasing the number of plates increases proportionally the size of a device. Specialized handling equipment such as Thermo Scientific’s Hand Manipulator system can be used to manipulate large plate stacks.
As happens with traditional plates operated in batch mode, culture medium must be replaced manually and periodically in plate stacks to provide growing cells with sufficient nutrients. This cyclic process step causes repeated shifts in process parameters such as pH, temperature, and nutrient and metabolite concentrations, all of which can lead to cellular stress (10–12). Such sudden shifts in process parameters are eliminated by the Integrity Xpansion system’s perfusion- mode operation using dedicated equipment. It offers a huge amount of surface area for cell attachment and reduces human intervention. However, the large numbers of cells growing within each culture chamber demands a very efficient oxygenation system. But in the Xpansion system, aeration occurs by gas diffusion through the wall of a central silicon tube placed at the center of a cylindrical chamber. Using a high flow speed during medium recirculation is one method that may overcome associated dissolved-oxygen limitations (13).
Hollow Fibers
The culture chamber of a hollow-fiber bioreactor comprises a number of hollow fibers bundled together and encased within a cylindrical cartridge. The structure of that bundle is such that the lumina of all fibers are connected to an inlet port at one end and an outlet port at the other end. The outer space (limited by the cartridge wall) is connected to a different set of inlet and outlet ports. Cells grow on the outer surface of the fibers while fresh medium circulates through the lumina in a continuous perfusion mode (14). This clever system — exemplified by Fiber Cell Systems and Biovest International, among others — has been successfully used to produce human influenza A virus (15). Such high cell densities are achieved within the cartridge that periodical debulking is necessary to prevent system clogging (16), and uneven distribution of culture conditions within cartridges has been reported (17, 18).
Roller Bottles
Roller bottles (e.g., Greiner BioOne’s CellMaster and other bottles by Thermo Scientific) have proven to be a sound solution for adherent cell culture. They have been used both to produce cells and to collect the culture medium in which growing cells have expressed target molecules (19). Such successes are a consequence of improved medium use combined with an increase in culture surface area in an easy-to-handle device. Gas transfer is extremely efficient in roller bottles because of their high mass-transfer coefficient, which results from a large contact area between the gaseous phase and a thin liquid-medium film on the wall of each bottle.
Although scaling up rolling bottles is a widespread approach in the biopharmaceutical industry, it does not seem to be an optimum approach. The strategy is as plain as using more bottles as necessary. Conditioned laboratories full of rollers (“roller bottle farms”) can end up accommodating huge numbers of bottles (in the thousands). Increasing roller-bottle diameter instead is not very sensible; to obtain 10 times as much inner area as in a 13-cm diameter bottle, you would need one with a 1.3-m diameter. Just imagine the size of the roller to handle such bottles!
Rolled Membranes
Instead, you might easily introduce a 2.5-m long rolled membrane within a traditional 13-cm diameter roller bottle (the tighter the spiral packing, the longer the available membrane). Because both sides of such a membrane would be wetted (and most likely colonized by a cell culture), you would obtain more than 10× the culture area of a bottle alone. This strategy has been proposed before (20, 21), but no commercial products are yet on the market based on such technology.
Most likely, this lack of adherent-cell culture devices based on rolled membranes comes down to two facts: A liquid flow and distribution system needs to ensure that culture medium is evenly distributed along each membrane. The massive cell population growing within such a device when a rolled membrane is fully colonized would demand continuous medium replacement and gas exchange to meet its metabolic demands. Therefore, a rolled membrane ideally should rotate while the culture chamber surrounding it remains stationary to prevent entanglement of supply and exhaust lines for media and gas.
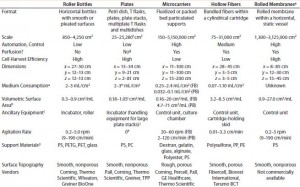
Table 1: Comparing features of cell culture devices based on different strategies for increasing productivity; only five strategies are considered here. Presented values are based on data reported publicly on the websites of companies listed; FB = fixed bed, PB = packed bed.
Meeting the First Challenge
Table 1 summarizes our findings on the different strategies for increasing cell culture surface area in light of the requirements put forward by Challenge #1. Following careful consideration of its findings, the BoB team understands that despite the achievements of current devices, no single system is yet automated and efficient enough for culturing adherent cells in different biopharmaceutical applications. The team has concluded that, provided that a uniform liquid-distribution and automated culture medium and gas replacement system were available, the best option to meet Challenge #1 would be rolled membranes. Respondents to a 2014 market survey (see the “Open Design Initiative” box) support this conclusion.
To prove that hypothesis, the BoB team has built several prototype iterations. The first version was promising but far from optimum. Ultimately the team has developed a rolled membrane shape that provides >10 times the surface area of a regular roller bottle intended to contain it. Upon rotation, medium distribution within the device is gentle and uniform throughout the whole membrane. You can find a video online that shows liquid flow on this rolled membrane at www. boltonbioreactor.com/about-the-bob-project/the-bolt-on-bioreactor/ challenge-1.html.
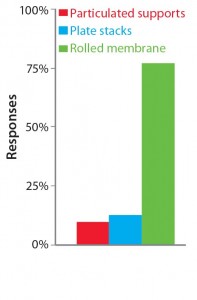
The Open Design Initiative: The BoB team has its own views on addressing the four challenges that must be met in designing a future standard solution for adherent cell culture. Market opinion is most important, however. So the team is conducting a series of surveys to provide insight on design features that would best suit market needs. A public survey conducted in May and June of 2014 asked what the best geometry would be to overcome Challenge #1. Results are shown above.
References
1 Bolt-on Bioreactor: Automated Adherent Cell Culture, 2014; www.boltonbioreactor.com.
2 Wang Y, Ouyang F. Bead-to-Bead Transfer of Vero Cells in Microcarrier Culture. Cytotechnol. 31(3) 1999: 221–224.
3 Nilsson K. Microcarrier Cell Culture. Biotechnol. Gen. Eng. Rev. 6(1) 1988: 404–439.
4 Cherry RS, Papoutsakis ET. Physical Mechanisms of Cell Damage in Microcarrier Cell Culture Bioreactors. Biotechnol. Bioeng. 32(8) 1988: 1001–1014.
5 Venkat RV, Stock LR, Chalmers JJ. Study of Hydrodynamics in Microcarrier Culture Spinner Vessels: A Particle Tracking Velocimetry Approach. Biotechnol. Bioeng. 49(4) 1996: 456–466.
6 Drivdal M, Broström G, Christensen KH. Wave Induced Mixing and Transport of Buoyant Particles: Application to the Statfjord A Oil Spill. Ocean Sci. Discuss. 11, 2014: 1265–1300.
7 Meuwly F, et al. Oxygen Supply for CHO Cells Immobilized on a Packed-Bed of Fibra-Cel Disks. Biotechnol. Bioeng. 93(4) 2006: 791–800.
8 Oh SKW. Suspension and Simulated Microgravity Cultures on Human Embryonic Stem Cells. Stem Cells: From Bench to Bedside, Second Edition. Bongso A, Lee EH, Eds. World Scientific Publishing: Singapore, 2011: 113–126.
9 Rowley J, et al. Meeting Lot-Size Challenges of Manufacturing Adherent Cells for Therapy. BioProcess Int. 10(3) 2012: S16–S22.
10 Krampe B, Al-Rubeai M. Cell Death in Mammalian Cell Culture: Molecular Mechanisms and Cell Line Engineering Strategies. Cytotechnol. 62(3) 2010: 175–188.
11 Varley J, Birch J. Reactor Design for Large Scale Suspension Animal Cell Culture. Cytotechnol. 29, 1999: 177–205.
12 Trummer E, et al. Process Parameter Shifting: Part I. Effect of DOT, pH, and Temperature on the Performance of Epo-Fc Expressing CHO Cells Cultivated in Controlled Batch Bioreactors. Biotechnol. Bioeng. 94(6) 2006: 1033–1044.
13 Roberts I, et al. The Importance of Using Small Scale Bioreactor Mimics to Scale up Human Embryonic Stem Cell Culture. University College London poster; www.atmi.com/ls-assets/pdfs/ bioreactors/xpansion/SmallScaleBioreactor MimicstoScaleupHumanEmbryonic.pdf.
14 Whitford WG, Cadwell JJS. Interest in Hollow-Fiber Perfusion Bioreactors Is Growing. BioProcess Int. 7(9) 2009: 54–63.
15 Tapia F, et al. Production of High-Titer Human Influenza A Virus with Adherent and Suspension MDCK Cells Cultured in a Single-Use Hollow Fiber Bioreactor. Vaccine 32(8) 2014: 1003– 1011.
16 Preechapornkul P, et al. Optimizing the Culture of Plasmodium falciparum in Hollow Fiber Bioreactors. Southeast Asian J. Trop. Med. Public Health 41(4) 2010: 761–769.
17 Tharakan JP, Chau PC. A Radial Flow Hollow Fiber Bioreactor for the Large-Scale Culture of Mammalian Cells. Biotechnol. Bioeng. 28(3) 1986: 329–342.
18 Piret JM, Devens DA, Cooney CL. Nutrient and Metabolite Gradients in Mammalian Cell Hollow Fiber Bioreactors. Can. J. Chem. Eng. 69, 1991: 421–428.
19 Kunitake R, et al. Fully Automated Roller Bottle Handling System for Large Scale Culture of Mammalian Cells. J. Biotechnol. 52(3) 1997: 289– 294.
20 Noteboom WD. Roller Culture Bottle Insert. US Patent 3,941,661. University of Missouri: Columbia, MO, 2 March 1976.
21 Johnson LR, Sapatino BV. Multiple Interior Surface Roller Bottle. US Patent #4,317,886. Becton, Dickinson, and Company: Paramus, NJ, 2 March 1982.
Marcos Simón, PhD, is founder of the Bolt-on Bioreactor Project, Technology Park, Mikeletegi 56, 20009 San Sebastian, Spain; marcos.simon@boltonbioreactor.com; www.boltonbioreactor.com.