Use of disposable bioreactors in the biopharmaceutical industry has increased gradually over the past several years in pilot, clinical, and production scale facilities (1–4). Reduced time to market in today’s drug industry has created a need for cost-effective development and production strategies as well as manufacturing flexibility. When compared with traditional stainless steel equipment, disposable bioreactor and mixing systems have smaller space requirements, are portable, and come presterilized to eliminate the need for preuse sterilization procedures such as steam-in-place (SIP).…
Upstream Processing
Process Development of Microbial Plasmid DNA: Fast-Tracking with Modular Single-Use Minibioreactors
There has been a rapid rise in the number of positive clinical outputs from clinical studies based on gene and cell therapies. This is in addition to the licensing of products such as GlaxoSmithKline’s Strimvelis ex-vivo stem-cell therapy for treatment of severe combined immunodeficiency caused by adenosine deaminase deficiency (ADA-SCID) in 2016 (1) — has led to an increase in demand for therapeutic vector manufacturing capabilities. Viral vectors are being used for an increasing range of conditions, including monogenetic conditions.…
How to Set Up a Perfusion Process for Higher Productivity and Quality
Biotherapeutic proteins usually are produced by either fed-batch or perfusion processes. Perfusion manufacturing can provide much higher levels of productivity than fed-batch systems can, thereby reducing production costs. A 2013 study showed that perfusion is more cost effective than fed-batch processes for most combinations of titers and production volumes (1). Moreover, because a perfusion process is much closer to steady state than is a fed-batch process, it often produces a more consistent product — especially for molecules that are sensitive…
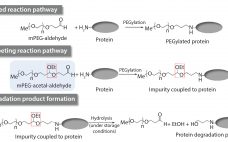
Setting Raw-Material Specifications Using Prediction Models: Determination of a Specification Limit for a Raw-Material Impurity in mPEG-Aldehyde
Impurities related to raw materials used for bioproduction can be inadvertently introduced into a manufacturing process, causing potential failure to meet in-process controls or release specifications. Unexpected impurities also can reduce yield and affect the quality, safety, and effectiveness of a final product (1). Raw-material impurities can originate from starting components or reagents used in manufacture. They can be generated in situ during synthesis or as degradation products. Impurities also can result from improper handling, packaging, and storage. Identification and…
Simplification of Fed-Batch Processes with a Single-Feed Strategy
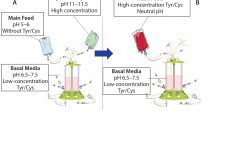
Chinese hamster ovary (CHO) cells commonly are used to produce recombinant proteins such as monoclonal antibodies (MAbs) for research, diagnostic, and therapeutic purposes. Culture processes typically rely on a fed-batch approach in which a basal medium enables initial cell growth. Concentrated feeds are used to prevent nutrient depletion, thereby extending culture duration and improving cell growth, viability, and protein titer. A neutral pH feed is desirable because culture pH should remain stable after feedings. The extremely low solubility of l-tyrosine…
Design and Performance of Single-Use, Stirred-Tank Bioreactors
Single-use components and systems have been incorporated into many bioprocesses as an alternative to cleanable, reusable systems. A wide range of publications have detailed the reasons for this trend toward a single-use approach. Justification in many cases comes from process-specific benefits such as increased manufacturing flexibility — especially for contract manufacturing organizations (CMOs) — enhanced sterility assurance, elimination of cleaning, reduced capital investment, faster processing times with increased productivity, faster start-up, and other benefits (1). One critical factor in the…
Continuous Cell Culture Operation at 2,000-L Scale
In the biopharmaceutical industry, continuous manufacturing is often cited as a method for increasing the productivity of bioprocesses (1). Compared with batch processing, it has the potential to enable production of more product within a smaller facility footprint — while improving product quality, particularly for sensitive and unstable molecules. Investigation into continuous methods is taking place for both upstream and downstream operations. For the full benefit of continuous processing to be realized, an argument has been made that cell culture,…
Taking Medium and Feed Development Beyond Maximizing Protein Titer to Optimizing Glycan Distribution and Simplifying Process Scale-Up
This webcast features: Serena Fries Smith, Process Science Manager, Thermo Fisher Scientific In the early 2000s when many processes were struggling to achieve 1 g/L, maximizing titers was the industry’s biggest challenge and was essential to having favorable cost of goods and an economically viable product. Over the last 1–15 years, the industry has made significant advances in media and feeds. Due to these advancements, today a standard fed-batch process can typically achieve 3 g/L and some processes are achieving…
Rapid Development of High-Quality, Robust Mammalian Cell Culture Manufacturing Processes
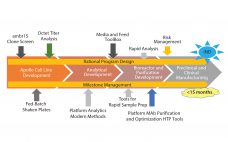
With increasing industry emphasis on providing both rapid and robust processes, companies are reaping the benefits of new tools for risk management and process analytical controls. As a current example of these approaches, Fujifilm Diosynth scientists have accelerated the development process from gene to finish by shortening the timeline, incorporating quality by design (QbD) principles, and designing the process to be as robust as possible. When the Apollo mammalian expression cell line was introduced three years ago, the time from…
Performance Qualification of a Single-Use, Stirred-Tank Bioreactor with CHO-S Cell Culture
The increasing role and importance of cell culture in biophamaceutical manufacturing has led to considerable research and development (R&D) into bioreactor design and performance in recent years. As a result, a greater understanding of bioreactor fluid dynamics and critical physical parameters is now essential to maximize cell growth and productivity. Stirred-tank bioreactors are especially important in this development process because of their favorable properties in areas such as mixing efficiency and homogeneity, energy transfer, and scalability. The design and manufacture…