This report explains how a wall pass-through system that incorporates Single-Use components can help speed and streamline operations, reduce contamination risks and maintain isolated environments.
Manufacturing
Efficacy and Utility of New EXP-Pak Closed-System Disposable Cell Expansion Bags Designed for Cell Therapy Applications
Clinical cellular therapy applications often times require a cell expansion or maturation step prior to use. Traditionally, cell expansion or cell culture is performed in “open” systems including multi-well culture dishes or tissue culture flasks. These “open” steps present risks and are not ideal for larger-scale manufacturing. The new EXP-Pak™ cell expansion container offered by Charter Medical, Ltd. is a closed-system, gas permeable bag intended for expansion and culture of non-adherent cells. The data demonstrate that the EXP-Pak™ cell expansion…
Industry Experts Convene in New York to Discuss Latest Innovations: A BPI Special Report
As the biopharmaceutical industry continues to mature and grow, so too does the need to educate a broader audience of biopharmaceutical professionals interested in hearing, understanding, and applying the latest science and technology trends that support and in many cases are transforming today’s bioprocesses. To reach this extended and engaged audience, BioProcess International created the BPI Theater Series: a live, interactive program that provides bioprocessing content to traditional, noncore biopharmaceutical conference programs. It provides attendees with the opportunity to interact…
Higher-Order Structure Comparability: Case Studies of Biosimilar Monoclonal Antibodies
Great successes for monoclonal antibody (MAb)–based biologics over the past decade have provided many valuable options for patients combating some of the most serious diseases in the world, including cancer and autoimmune diseases. MAbs and antibody–drug conjugates (ADCs) are among the fastest growing biologic segments in development, with hundreds of candidates currently under clinical study. Meanwhile, society is facing the challenge of increasingly higher costs in healthcare including the cost of pharmaceuticals. With an aging population in many parts of…
Rapid Development and Scale-Up Through Strategic Partnership: Case Study of an Integrated Approach to Cell-Line and Process Development for Therapeutic Antibodies
Over the past decade, monoclonal antibodies have become mainstream therapeutics for treating a broad range of conditions from autoimmune disorders to cancer. Part of this evolution is increasing time and cost pressure on biopharmaceutical companies to bring new drugs to market 1, 2. Additionally, companies now routinely engineer and screen molecules for developability and manufacturability during discovery before selecting a final candidate molecule. The biosimilar development paradigm also demands significantly more bioanalytical analysis during initial cell-line and process development. Thus,…
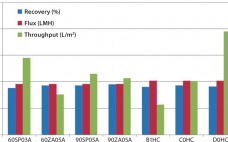
Strategies to Mitigate Technology Transfer and Clinical Manufacturing Risks: Downstream Purification Case Studies
Since the early 1980s, biotechnology products have been a fast-growing sector. They now occupy a significant portion of biologic drugs approved by regulatory authorities around the world every year. Among the approved biologic drug products, as well as those still in clinical testing, many are manufactured by contract manufacturing organizations (CMOs). Sponsor companies often transfer their developed process and process knowledge to CMOs for manufacturing of materials for toxicology and clinical studies. Drug Product Development Figure 1 illustrates the usual…
Begin By Thinking of Your Goal
The biopharmaceutical industry is experiencing a surge of collaborations among large and small companies seeking to develop new drug candidates. Often, such efforts have been a result of a merger or acquisition. But other factors also are pushing the rise in collaborations, including dwindling drug pipelines, increasing generics and biosimilars, rising costs of drug development, and changing regulations that are already complex. High costs of drug development in particular have created greater risks. That is especially true for small biotechnology…
Seeking the Next Generation of Single-Use Technologies
Recent trends in biomanufacturing technology and the biopharmaceutical market are driving the increased adoption of single-use (SU) manufacturing systems. From the demand side, the biopharmaceutical industry’s focus on niche and rare diseases with relatively small patient populations is pushing for smaller, more flexible biomanufacturing capacities than have been needed in the past. The entry of many companies into biosimilars development also is leading to fragmentation and dispersion of manufacturing capacity. Changes on the supply side caused by technological advances have…
The Importance of a One-Stop-Shop-6 Questions to Ask Your Fluid Management Component Supplier
A one-stop-shop fluid management component supplier assures quality, streamlines processes, and limits costs. To be certain you receive the preeminent and safest single-use component, take time to evaluate your fluid-management supplier or a supplier under consideration by asking the 6 important questions we pose in this whitepaper. Your supplier’s answers will help determine if the company is a steadfast partner and one-stop-shop committed to helping you pass FDA validation. You also will learn your supplier’s willingness to provide: the best…
Cell Therapy Will Transform the Future of Medicine
The third annual IBC Cell Therapy Bioprocessing conference was held in Bethesda, MD, on 21–22 October 2013. It brought pioneers in the development of cell-based therapies together with companies that have enabling technologies, such as bioreactors, cell culture media, and advanced monitoring software. After the conference, I discussed the highlights and key themes coming out of the event with Dr. Phil Vanek, general manager of cell bioprocessing at GE Healthcare Life Sciences in Westborough, MA. Also an instructor for advanced…