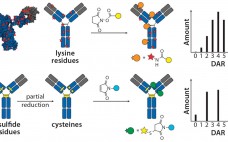
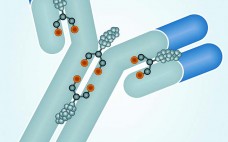
Monoclonal antibodies have dominated the biopharmaceutical market for over two decades. Few people doubt that their future success in fields such as oncology, inflammation, and autoimmune diseases owes much to the development of antibodies conjugated to cytotoxic drugs. Like all great innovations, this is a breathtakingly simple concept: Combine the targeting specificity of an antibody with a small-molecule drug as an effector component joined to that antibody by means of a small chemical or peptide linker, and you have a…
Manufacturing
Bioprocessing Challenges of Antibody–Drug Conjugates
Development of highly potent active pharmaceutical ingredients (HPAPIs) is clearly a pharmaceutical industry trend. Highly potent drug products involve active agents and APIs that are so potent therapeutically (or simply just outright toxic) even in small dose that special precautions are required during their manufacture — particularly when handling the active agents. Such requirements include maximal containment and isolation of the process stream. Worker exposure and environmental release clearly pose problems. The necessity and intensity of containment efforts with HPAPIs…
The Next Step in Homogenous Bioconjugate Development: Optimizing Payload Placement and Conjugate Composition
[Audio Recording] Bringing a new biologic drug to market is a long and expensive process, with research and development (R&D) cycles that can span up to 15 years and may cost over a billion dollars. Biologic drug development also involves significantly more complex manufacturing and CMC components than does development of small molecules. Nonetheless, the pharmaceutical industry is increasingly shifting its R&D efforts to focus on biologic drugs. According to a recent report from Tufts Center for Study of Drug…
Bioconjugation Reaction Engineering and Kinetics Simulation
Bioconjugates represent an important and growing class of pharmaceuticals that include PEGylated proteins, vaccines, and antibody-drug conjugates (ADCs) (1–8). Numerous protein conjugation techniques exist (9). Among the more important conjugation chemistries used for protein therapeutics are N-hydroxysuccinimide (NHS), aldehyde, and maleimide (10–13). To date, process development of industrial biopharmaceutical conjugation reactions has largely been empirical in nature. Typically, many experiments testing different reaction parameters are required to identify optimal process conditions. In some instances, nonmechanistic statistical models can be used,…
Fine-Tuning ADCs for Best-in-Class Therapeutics
Antibody–drug conjugates (ADCs) use the targeting ability of a monoclonal antibody (MAb) to deliver a highly biologically active drug to diseased cells while sparing healthy cells, creating potent and effective therapies. This emerging class of novel drugs currently focuses almost exclusively on cancer treatment. Two blockbuster ADCs — brentuximab vedotin (Adcetris from Seattle Genetics) for treatment of rare lymphomas and ado-trastuzumab emtansine (Kadcyla from Genentech/ Roche, manufactured by Lonza) for treatment of HER2-positive metastatic breast cancer — have improved treatment…
Predicting Aggregation Propensity and Monitoring Aggregates in ADCs
Antibody–drug conjugates (ADCs) are monoclonal antibodies coupled to cytotoxic agents with stable linkers. ADCs travel to target cells, where the antibody binds to its antigen expressed on the cell surface. Upon binding, the full ADC can be internalized by a process called receptor-mediated endocytosis. That process is followed by lysosomal degradation of ADC complexes, which ultimately leads to release of the cytotoxic agent and apoptosis of the target cell. Drugs used in ADCs can be up to a thousand times…
Technology Advances Enable Creation of Better ADCs
Antibody–drug conjugates (ADCs) for treatment of cancer combine the tumor-targeting properties of antibodies with the cell-killing properties of cytotoxic drugs. By targeting a drug to a tumor, it is possible to reduce systemic toxicity and thereby enable administration of drugs that are otherwise too toxic to be effective therapies. Although the concept of an ADC is simple, in reality developing an effective treatment is somewhat more challenging. Whether an ADC has sufficient efficacy at a tolerable dose depends on four…
Using a CMO for Your ADC: Access Analytical and Manufacturing Platforms, Specialized Facilities, and Expertise
[Audio Recording] Antibody–drug conjugates (ADCs) are an exciting new area of therapeutics. They bring the “magic bullet” that was promised by Paul Ehrlich over a hundred years ago to reality by targeting cancer cells to deliver chemotherapies without poisoning a patient’s whole body. ADCs offer a promising form of therapy by providing higher safety margins than traditional chemotherapeutics alone, and they make selectivity possible. We should be able to personalize a therapy to the specific cancer expressed in a given…
Designing Single-Use Solutions for the Future: A Conversation with Christel Fenge
Recently, BPI publisher Brian Caine and editor in chief Anne Montgomery had the opportunity to talk with Christel Fenge, Sartorius Stedim’s VP of marketing for fermentation technologies, in the Göttingen, Germany, Sartorius facility. They began by talking about fermentation technology, a topic that led them to touch upon a number of key issues in the biopharmaceutical industry. Fermentation Technology Caine: Because this special issue focuses on fermentation technology, let’s begin by talking about some recent technological improvements and what impact…
Robust and Convenient Single-Use Processing: The Superior Strength and Flexibility of Flexsafe Bags
With the increased use of disposable bioprocessing bags in all critical process steps of the biopharmaceutical drug production, there is a growing requirement for high-quality, robust, and easy-to-handle bioprocessing bags. The new generation of films and bags must combine multiple mechanical, physical, and chemical properties to make these products suitable and scalable for all processing steps in upstream, downstream, and final filling operations, including cell culture in rocking motion and/or stirred-tank, single-use bioreactors as well as storage, mixing, shipping, and…