Cell therapy is the injection of cellular material into patients. The injected cell-therapy product (CTP) usually consists of intact living cells. In recent years, cell therapies have evolved and matured, moving from academia to industry. That maturation is reflected in the number of open clinical trials that include the term cell therapy in their descriptions: To date, there are more than 8,700 open trials listed on the US National Institutes of Health’s online database (clinicaltrials.gov), most of which are in…
Manufacturing
Extracellular Vesicles Commercial Potential As Byproducts of Cell Manufacturing for Research and Therapeutic Use
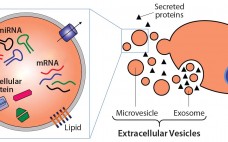
Extracellular vesicles (EVs) are emerging as a potential alternative to some stem-cell–derived therapeutics (1, 2). Sometimes called exosomes, they are small, secreted vesicles that can possess similar therapeutic mechanisms to whole cells, possibly representing the active pharmaceutical ingredient. In the past 15 years, academic and industry interest in EVs has exponentially increased as mounting evidence demonstrates their role in physiology and pathology as well as their therapeutic potential. In light of growing efforts in using EVs for research and therapy,…
Maximizing PMBC Recovery and Viability: A Method to Optimize and Streamline Peripheral Blood Mononuclear Cell Isolation, Cryopreservation, and Thawing
The quality of peripheral blood mononuclear cells (PBMCs) isolated from whole blood has a significant impact on their subsequent analysis. Maximizing recovery, viability, and functionality of isolated PBMCs is essential to the reliability and consistency of downstream applications, particularly within cell therapy manufacturing. The standard method for purification of PBMCs is density-gradient centrifugation. It requires precise layering of whole blood over a density medium (e.g., Ficoll polysaccharide reagent from GE Healthcare), with careful pipetting of the floating cell layer after…
The Potential Application of Real‑Time Release Testing for the Biomanufacture of Autologous Cell‑Based Immunotherapies
Cell-based immunotherapies (iTx) are emerging as a truly transformative therapeutic modality that is both complementary and convergent with existing regenerative medicine approaches, including gene therapy, cell therapy, and tissue engineering (Figure 1). Critically, iTx offer step-change improvements in efficacy compared with current standards of care (1) for a range of clinical indications and unmet therapeutic needs — particularly oncology. The clear efficacy of iTx is in contrast with some previous regenerative medicine approaches, including early mesenchymal stem cell (MSC) therapies…
Are You Ready for a Tech Transfer? Part 1: Challenges and Critical Factors for Success in Cell Therapy Development
Cell therapies offer enormous promise for treatment of a range of conditions by replacing damaged tissue or leveraging the body’s own resources to heal itself. Not surprisingly, the cell therapy industry is growing rapidly and is poised to have a major impact on healthcare and disease treatment. The Alliance for Regenerative Medicine (ARM) has reported on the robust state of the industry, noting that revenue from cell-derived products grew from US$460 million in 2010 to $1.3 billion in 2013 (1).…
Outsourced Data Integrity: Are Short-Term Financial Gains Worth Long-Term Headaches?
Competitive pricing and continued cost pressures have contributed to the need for many US biopharmaceutical companies to outsource manufacture of active pharmaceutical ingredients (API) and finished products from countries with lower costs for labor, material, and equipment. The main benefit of doing so is lower costs of manufacturing with quality standards comparable to those found in the United States. India and China now account for 80% of API production. But those countries have received media attention because of biopharmaceutical manufacturing…
Rapid Development and Scale-Up of Biosimilar Trastuzumab: A Case Study of Integrated Cell Line and Process Development
Compared with that for new drugs, biosimilar development faces significantly condensed timelines from cell line to first-in-human (FIH) trials. A biosimilar development program needs to accelerate quickly toward preclinical and phase 1 studies; phase 2 studies typically are not required because dose response and other patient-treatment concepts are already established by the original, comparator medicine. Phase 3 studies typically are limited to fewer patients, which ultimately shortens overall timelines and costs. The key challenge remains: demonstrating comparability and high similarity…
Toward Industry Standardization of Extractables Testing for Single-Use Systems: A Collective BPSA Perspective
Here we present a consensus position of the membership of the Bio-Process Systems Alliance (BPSA), the trade organization for the single-use industry based in Washington, DC. BPSA’s membership includes 48 corporate and institutional entities, among them component suppliers, systems integrators, end users, and independent testing laboratories. Consensus within this membership is reached through an official ballot of representative voting members, as provided for in the organization’s by-laws. The position outlined below was approved by such an internal consensus-balloting process. Building…
Extractables Profiles: A Comprehensive Approach Produces Long-Term Results
Biopharmaceutical manufacturers spend years developing and testing new drug and biologic products to ensure their efficacy, safety, and usability for patients. Such knowledge is extremely valuable, but those same principles often get overlooked in selection of packaging and delivery systems. Decisions on packaging and delivery often are made almost as an afterthought. However, issues related to components such as extractables and leachables can affect patient safety and product quality. In addition, a lack of extractables and leachables data in filings…
The New Hybrid: Single-Use Systems Enabled By Process Automation
Much has been written in recent years about the union of single-use systems (SUS) and process automation. These two technology initiatives have been prevalent within biopharmaceutical manufacturing over the past decade and are two of the most predominant advancements in biomanufacturing. A basic survey of industry media and conference topics corroborates that premise (1). However, efforts to combine them remain in the early stages of technological fulfillment, with much work to be done in realizing their synergistic benefits. Here I…