Last month, Part 1 of this discussion briefly described the regulatory landscape for developing biosimilar therapeutic monoclonal antibodies (TMAbs). We identified certain specific structural components of TMAb drug substances that warrant particular attention because alterations to them are likely to affect therapeutic safety and effectiveness. Now we conclude by considering whether studies of reference materials can further the development of analytical industry standards to ensure comparability of putative biosimilar TMAbs with innovator TMAbs. We suggest that the time is right…
Manufacturing
Special Report on Antibody-Drug Conjugates: Technical Challenges and Opportunities
Among the emerging targeted therapies in biotechnology, antibody–drug conjugates (ADCs) hold a unique position. An ADC consists of a monoclonal antibody (MAb) with affinity to tumor cells, a cytotoxic small-molecule payload, and a linker connecting the two. Together the MAb, conjugation chemistry, and cytotoxin increase the complexity of ADCs several-fold relative to unmodified MAbs — and exponentially relative to chemotherapies. Viewing ADCs as hybrids of antibody- and chemotherapy-based cancer therapies is tempting. That description applies chemically and structurally, but ADCs’…
Engineering Tissues with Bioprinting
Commonly referred to as three-dimensional (3D) printing, additive manufacturing encompasses a set of technologies that fabricate objects in an additive way, layer by layer, rather than conventional means of fabrications that generally subtract unwanted material from a larger block. Precise control over material placement allows 3D printing to fabricate objects that otherwise would not be manufacturable. Although many of these technologies have been around for two or three decades, recently they have received a significant amount of attention from industry,…
Progress Toward Commercial Scale and Efficiency in Cell Therapy Bioprocessing
Regenerative medicine includes both cell and gene therapies. Currently 672 regenerative medicine companies operate around the world, and 20 products have been approved by the US Food and Drug Administration (FDA). Of 631 ongoing clinical trials by the end of 2015 (1), over 40% are in oncology, followed in prominence by cardiovascular and infectious diseases. Here I focus on gene and cell therapy bioprocessing in which the final products delivered to patients are cells. Cell therapies are either autologous (derived…
Manufacturing Plasmid DNA: Ensuring Adequate Supplies for Gene and Cell Therapies
The concept of gene therapy is far from new, with initial studies performed over 20 years ago (1). However, in the past few years an explosion of interest in this area has gone beyond initial regenerative approaches using viral vectors. Interest is now moving increasingly into potential use of T cells modified using recombinant viral vectors for immunotherapy applications. Such therapies are based on using either adenoassociated virus (AAV) or lentivirus (1), both vectors being frequently generated through transient expression…
Designing the Optimal Manufacturing Strategy for an Adherent Allogeneic Cell Therapy
Cell therapies (CTs) offer potential treatments for a wide range of medical conditions (1–6) by replacing cells, repairing tissues affected by either disease or damage (7), or delivering genetic or molecular agents that promote self-healing (8). CT research and development is continuously growing (9), with increasing numbers of CT candidates reaching phase 3 clinical trials (9–11). Developers aim to make products that can survive in a competitive landscape while complying with stringent regulatory requirements to control the quality and safety…
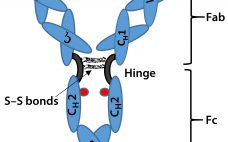
Biosimilar Therapeutic Monoclonal Antibodies: Gaps in Science Limit Development of an Industry Standard for Their Regulatory Approval, Part 1
Biosimilars are biologically derived pharmaceuticals intended to have clinical similarity to a legally marketed innovator product when that product’s patent or market exclusivity has expired. By contrast with generic small-molecule drugs, clinical performance of a biologic pharmaceutical is a function of its structural complexity and higher-order structure (HOS). Biomanufacturing controls of such complex products cannot fully ensure chemical similarity between an innovator product and putative biosimilar because minor differences in chemical modifications and HOS can significantly alter a product’s safety…
Emerging Technology Trends in Biologics Development: A Contract Development and Manufacturing Perspective
For a contract development and manufacturing organization (CDMO), process development and manufacturing of recombinant proteins must be linked because of tight timelines driven by client expectations. Those are in turn driven by a need for rapid progression to clinical testing. Early in process development, the choice of raw materials needs to reflect existing supply chain and manufacturing infrastructure, but remain suitable for scaling up to meet future needs. One approach is to establish platform processes for a class of molecules…
Validation of Controlled Freezing and Thawing: A 9-L Bottle Study
Freeze–thaw processes affect the quality of biopharmaceutical proteins (1–13) and human cells (14). It has been reported that no method consistently controls freezing and thawing rates for biological formulations (1). My recent study refutes that claim with validated rate-controlled freezing and thawing of such formulations in 16-L single-use bags (15). The study reported herein presents a consistent method for controlled-rate freezing and thawing of bottled formulations. It also highlights the effect of load and container position on freeze rates. The…
Biopharmaceutical Contract Manufacturing Technology and Capacity Investments
The market for biopharmaceutical contract manufacturing has shown robust growth over the past few years. The continuing growth of biopharmaceuticals and the increase in new and novel drug projects entering clinical pipelines are fueling the market’s double-digit growth rate. Responding to these new demands, contract manufacturing organizations (CMOs) are expanding both their capabilities and capacities. Background Information presented here draws from recent interviews with six executives at biopharmaceutical contract manufacturing organizations, and from HighTech Business Decisions’ latest report, Biopharmaceutical Contract…